What results when a species is OXIDIZED?
2 Answers
Well, cations are oxidation products.......
Explanation:
And thus for an element, we would write......
And anions, which typically result from non-metals, are reduction products....
Salts result from the stoichiometric combination of anions and cations......i.e.
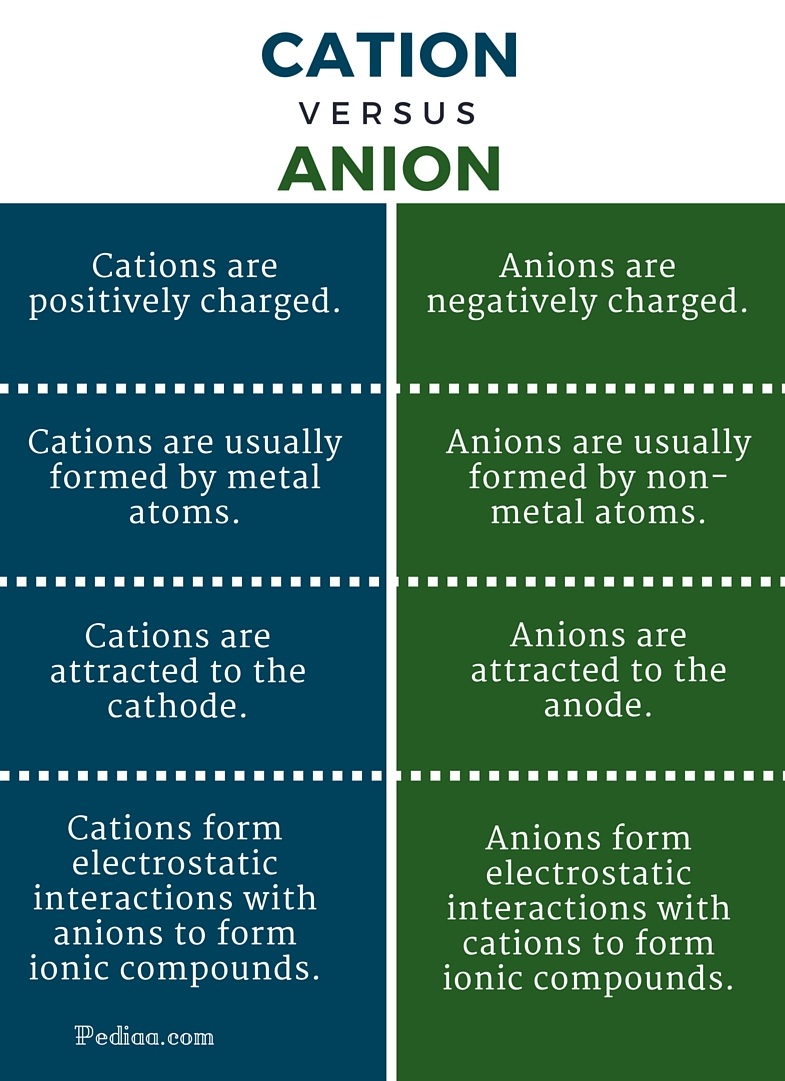
Cations (positive charge), anions (negative charge)
Explanation:
The difference between a cation and an anion is the net electrical charge of the ion. Ions are atoms or molecules which have gained or lost, one or more valence electrons (electrons on their outer shell) giving the ion a net negative or positive charge.
Cations lose one or more valence electrons. Therefore, they have a net positive charge .
Anions gain electrons, means they gain a net negative charge.
Example of cations:
#Na^+1# #Ca^+2# #Al^+3#
Example of anions:
#Cl^-1# #O^-2# #N^-3#


