Is the nonbonding orbital of "NH"_3 a p orbital?
1 Answer
MOSTLY true. The lone pair is in a molecular orbital that is similar to a
Consider this orbital diagram of
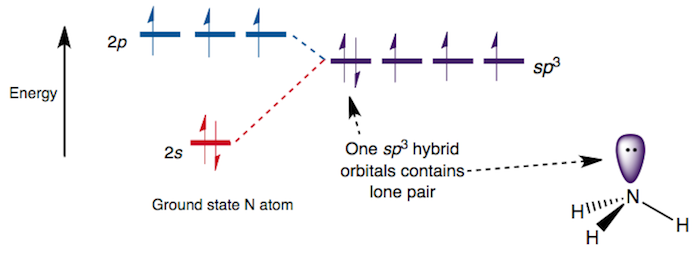
In a sense, I suppose the lone pair is in a
- In a hybridized treatment, one would place the nitrogen lone pair in an
sp^3 orbital, as seen in the above diagram. In this case, all four orbitals nitrogen uses for bonding are identical, with75% p character and25% s character. - In an unhybridized treatment, one would place the nitrogen lone pair in a molecular orbital with significant
p characteristics, and the molecular orbital would primarily belong to the nitrogen.
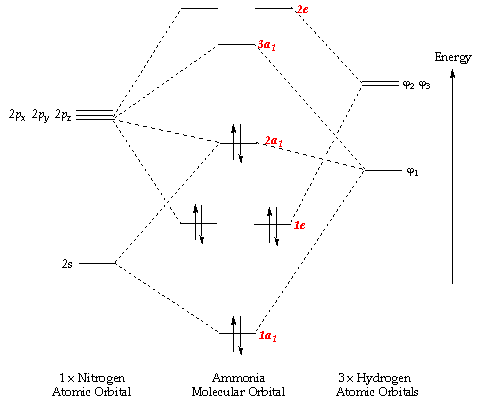
And in the above molecular orbital diagram, I would be referring to the molecular orbital labeled
2a_1 , which you can see involves interactions between more than one orbital.Therefore, it is not a pure, nonbonding atomic orbital.
Either approach leads to the conclusion that the orbital contains significant

