Your tool of choice here will be the Periodic Table, so start by grabbing one.
Now, notice that the electrons are said to occupy the 4p subshell. As you know, the coefficient added in front of the name of the subshell tells you the energy shell in which the subshell is located.
In this particular case, you know that these electrons are located in the "4th" energy shell. This implies that you're looking for two elements located in period 4 of the Periodic Table.
Now, the Periodic Table can be organized into blocks that correspond to the energy subshells in which a given electron is located.
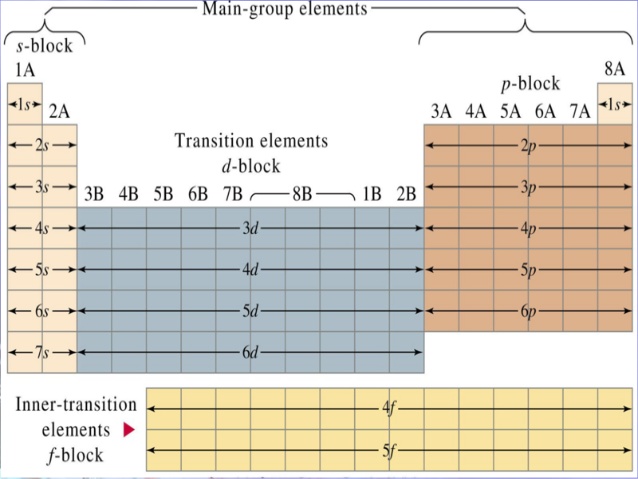
The p subshell corresponds to the p block, which includes all the elements in groups 13 through 18. The 4p subshell corresponds to the elements that have their higher-energy electrons in the 4p subshell.
This implies that the smallest possible value for the atomic number of an element that has its outermost electrons in the 4p subshell is 31 because this atomic number corresponds to the element located in period 4, group 13, i.e. to gallium, "Ga".
Similarly, the largest possible value for the atomic number of an element that has its outermost electrons in the 4p subshell is 36 because this atomic number corresponds to the element located in period 4, group 18, i.e. to krypton, "Kr".


