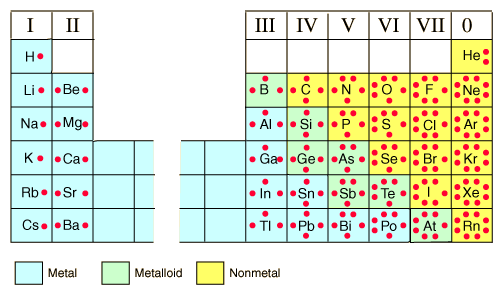
What groups on the periodic table have four unpaired valence electrons and one unpaired valence electron?
1 Answer
Dec 25, 2017
There are 4 unpaired electrons in the ground state of the atoms in group 14, and 1 unpaired electron in the ground state of the atoms in group 17.
Explanation:
There are 4 unpaired electrons in the ground state of the atoms in group 14, and 1 unpaired electron in the ground state of the atoms in group 17.
The image below shows the representative (main group) elements. Group 14 is IV, and group 17 is VII.