How do you use shorthand notation?
1 Answer
Dec 26, 2017
To use a noble gas in the electron configuration.
E.g.
Explanation:
It means to use noble gas configuration to shorten the electron configuration names.
We could use it for big elements like Uranium (
Instead of writing:
Uranium has an electron configuration of
We can write it with a noble gas, which shortens it immensely
From
we shorten it to just
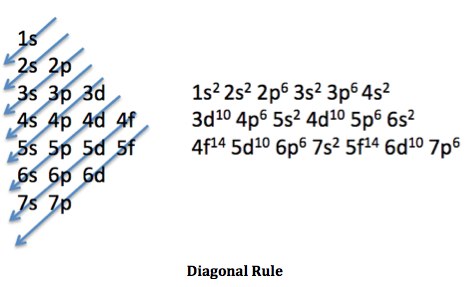
The trick is to use the closest noble gas to the element you are writing it with the e. configuration, and then continue with the pattern shown in this picture: