Question #67d83
1 Answer
Dec 30, 2017
There are three chiral centres in atropine sulfate.
Explanation:
I have marked them with asterisks in the diagram below.
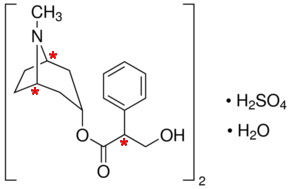
(Adapted from Sigma-Aldrich)
Carbon
Atoms
It is carbon

