Question #0442e
1 Answer
Feb 4, 2018
Na is bigger than Mg because of one less proton in the nucleus to attract the outermost electron cloud
Explanation:
See the image below
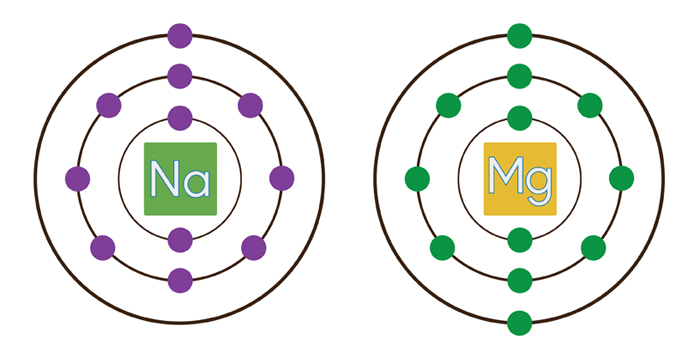
Na is bigger than Mg because of one less proton in the nucleus to attract the outermost electron cloud
See the image below
