What element in the fourth period is an exception to the Aufbau principle?
1 Answer
Copper and chromium
Explanation:
The aufbau principle states that electrons are placed in orbitals of lower energy levels before placing themselves in higher energy levels. It goes like this:
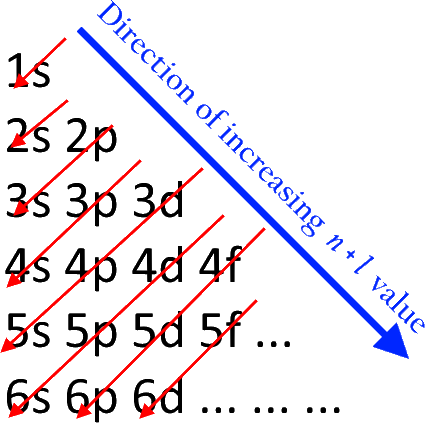
So first, we have
Writing down the electron configuration of vanadium, the element just before chromium, we have:
Obviously, chromium should be same, except that we have
Similarly, for nickel, the element just before copper, the config is:
For the same reasons as before, copper's config is:
It all ties up.

