What happens to the distance between energy levels at higher energy levels?
1 Answer
Mar 14, 2018
The distance shrinks. I.e the energy levels become closer or "converge" as it is often referred to.
Explanation:
According to the Bohr Atomic model (courtesy of Wikipedia) electrons are located at specific energy levels from the atomic nucleus. This is from evidence based on the Hydrogen emission spectrum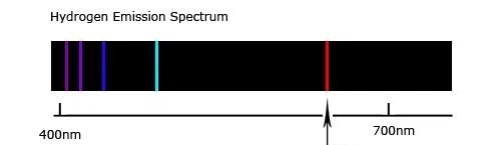 (Couretsy of Pratik Chaudhari on Quora.com)
(Couretsy of Pratik Chaudhari on Quora.com)
As seen in the diagram, the shorter wavelength emission lines, which correspond to the emission of more energetic forms of light, seems to grow closer and closer the shorter they get. The shorter wavelength a wave has, the greater energy it holds, therefore, this is evidence to suggest that electron energy levels converge at higher energy levels.

