How are chlorides made?
1 Answer
Mar 26, 2018
By chlorides, I assume you mean -chloride ionic compounds.
Explanation:
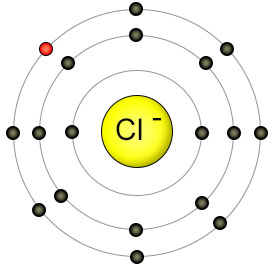
This gained electron (highlighted in red) can come from any electron-donating ion.
Let's take the most common chloride salt formed,
Sodium as an atom has one electron in its outermost 2s1 sub-orbital, hence the