What is ionic size?
1 Answer
May 24, 2018
As the name says on the tin, the ionic radius is the radius of the TYPICAL ion of a given atom...
Explanation:
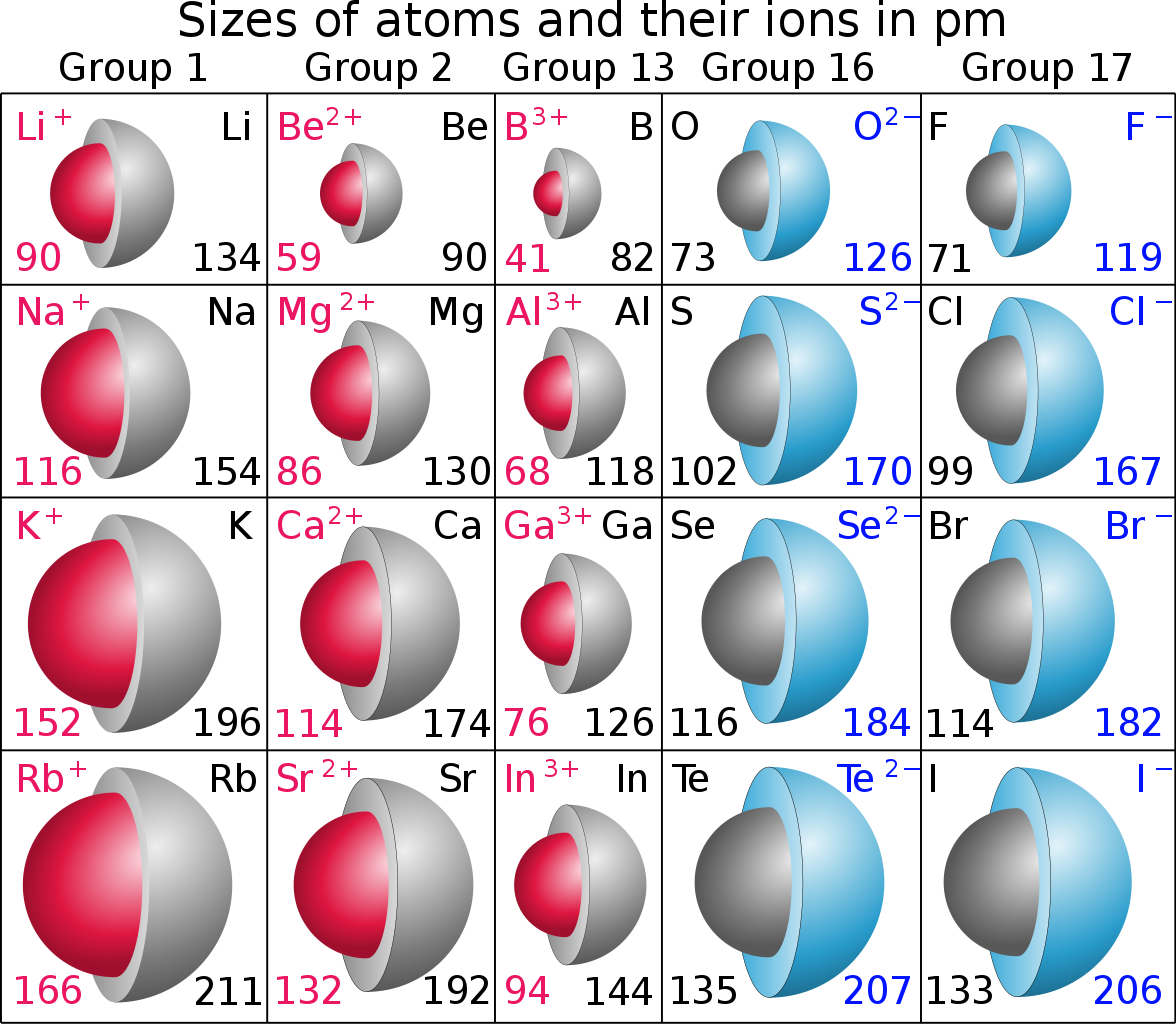
And as is typical, metals, electron-rich materials from the left and side of the Periodic Table tend to lose electrons to form cations, i.e.

