How would you explain ionic radii?
1 Answer
Let us begin by attempting to explain ATOMIC radii....
Explanation:
The atomic radius is REASONABLY defined by the orbit of the outermost electrons as it whizzes around the nuclear core. And we know (or should know) from first principles that ATOMIC SIZE decreases across a Period from left to right as we face the Table, but INCREASES down a Group. Why? Because incomplete electronic shells shield the nucular charge very ineffectively, and as
And upon oxidation, i.e. LOSS of ELECTRONS, electronic shielding is FURTHER compromised, and the radius of the CATION, should be substantially reduced with respect to the PARENT atom...
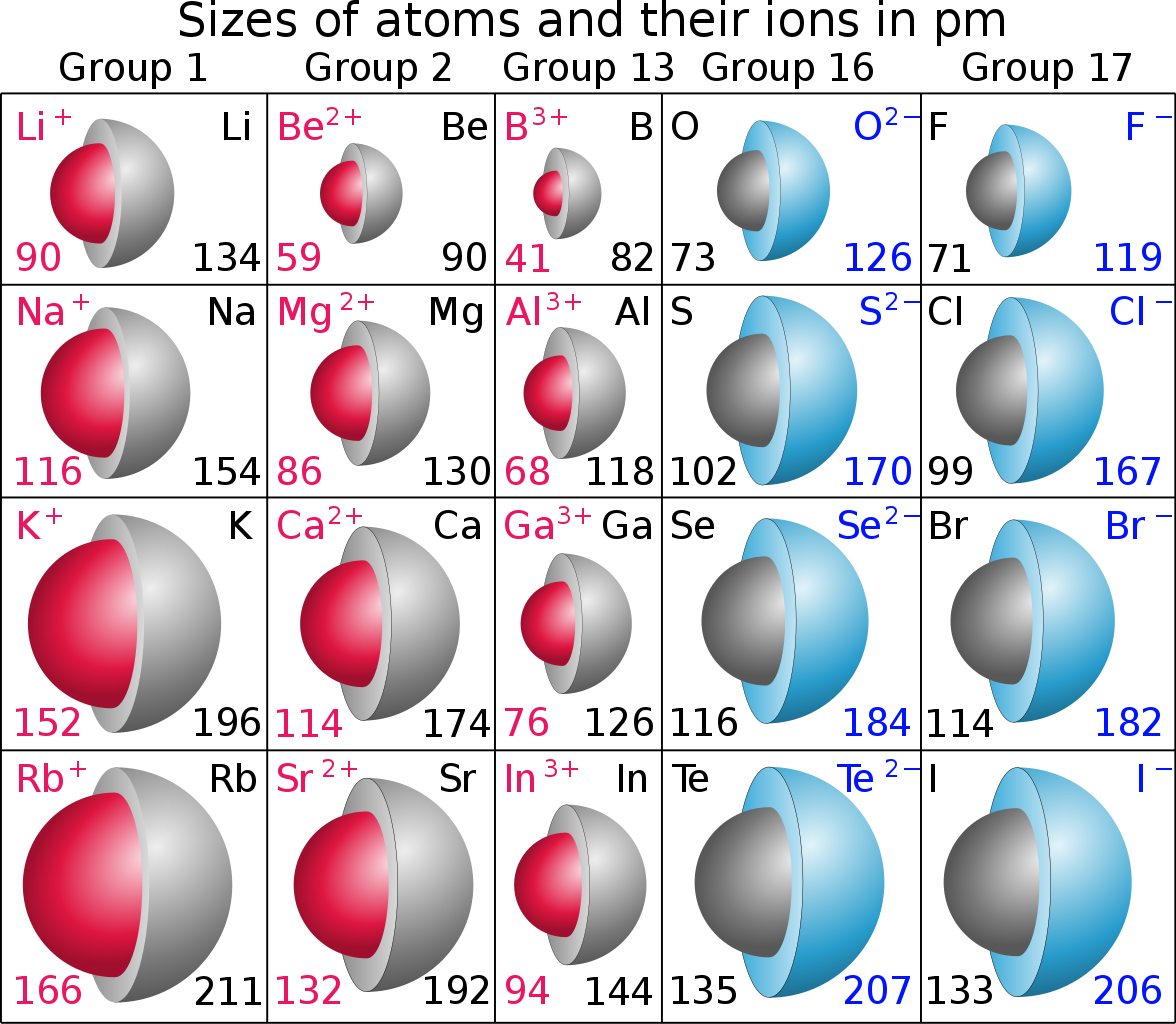
Looking at the parent metal atoms and metal cations, the red spheres, do the data support this rationale? The units are
On the other hand, non-metals from the right of the Table as we face it, have HIGHER unshielded nuclear charge, and therefore they tend to be oxidizing, i.e. they accept electrons to form negative anions and dianions. And if you look at elemental oxygen, and fluorine, the MOST oxidizing elements on the Periodic Table, CLEARLY their ions, i.e. ANIONS, are LARGE with respect to the parent atoms.... Upon reduction, the extra electrons strongly shield each other, and anionic radii ARE INHERENTLY larger..
And so we have attempted to explain the trends in ionic radii on the basis of oxidation and reduction... The former REDUCES the ionic radius with respect to the neutral parent, and the LATTER INCREASES ionic radius.... The contest between nuclear charge and shielding by other electrons, which rationalizes atomic size and Periodic structure, may also be invoked for discrete ions.....

