What are lone pairs and how are they represented in a Lewis dot diagram?
1 Answer
These are conceived to be pairs of electrons present on the central atom, that DO NOT participate in bonding....
Explanation:
And ammonia is a go to example....
For nitrogen,
And so we gots…
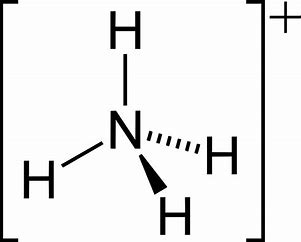
On other hand, the lone pair explains the basicity of the ammonia molecule. Ammonium ion,
And they are normally represented by a double-dot...alternatively we could try to draw the
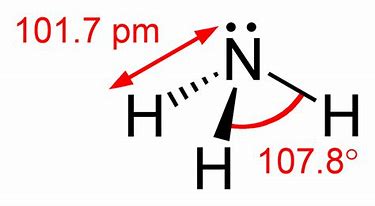
Note that ammonia is a rather potent donor, and as well as binding to a proton
Ammonium ion is more or less a regular tetrahedron...with