How does ionic radius change across the periodic table?
1 Answer
This is a hard question to address....
Explanation:
You know that ATOMIC size decreases across the Period, from left to right as we face the Table. Why? Well, atomic size is a function of (i) the nuclear charge
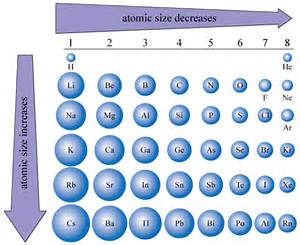
On the other hand, an ion may be produced by REDUCTION, to give an anion, or by OXIDATION, to give a cation, the which should be much smaller than that of the parent. A priori, we would expect that the ANION would have GREATER radius than its parent atom...and the PARENT ATOM would have GREATER radius than its daughter cation....given that atomic radius is defined by the radius of its valence electron.
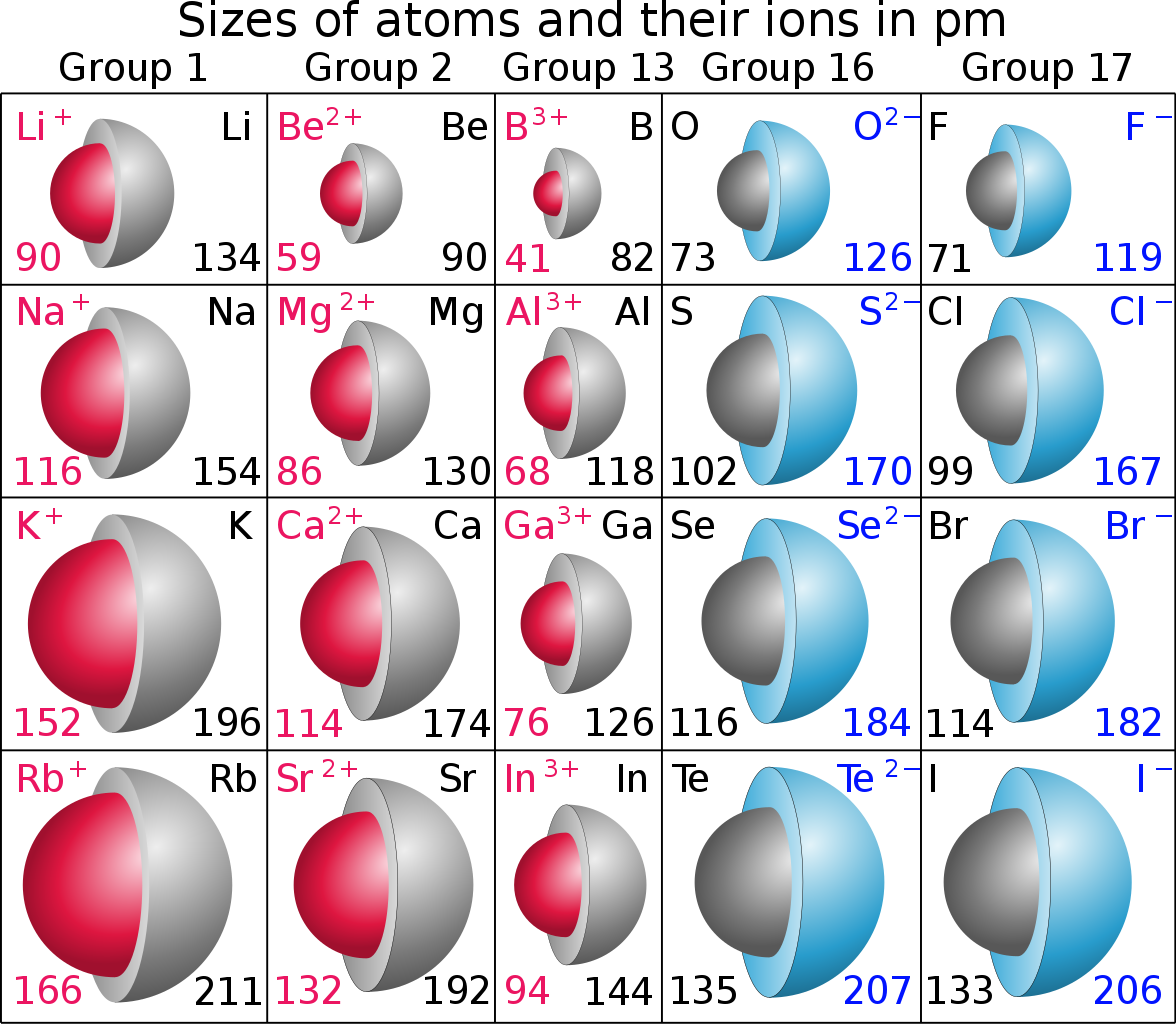
Units are in

