How are London forces affected by molecular size?
1 Answer
Jul 7, 2018
Well, consider the boiling points of the Noble Gases, a category which directly interrogates dispersion forces....
Explanation:
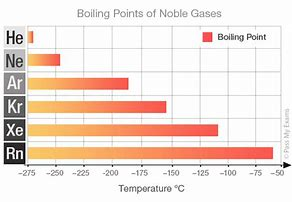
It is significant that the more electrons the Noble gas has, the HIGHER the boiling point. The larger the molecule, here the ATOM, the more electrons it has, and thus the GREATER the opportunity for dispersion forces to operate. And thus both radon, and xenon, many electron atoms express higher normal boiling points.

