What is the electron configuration of chlorine?
1 Answer
full ground state electron configuration:
abbreviated:
Explanation:
Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form.
We'll need to know how many sublevel is present in each energy level, and in turn, how many electrons each sublevel can accommodate.
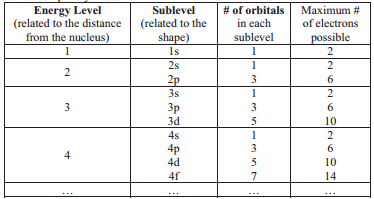
From the given table, for energy level 1, there's only 1 sublevel, which is called 1s. In it, there's only 1 orbital. Since 1 orbital can hold at most 2 electrons, therefore 1s can hold max 2 electrons .
For energy level 2, there are 2 sublevels, 2s and 2p .
- 2s, only has 1 orbital, therefore 2s at most will hold max of 2 electrons .
- 2p has 3 orbitals, and therefore can hold max of 3 x 2 = 6 electrons
For energy level 3, there are 3 sublevels, 3s, 3p and 3d.
- 3s, has 1 orbital, therefore, max 2 electrons .
- 3p, has 3 orbitals, therefore, max 6 electrons .
- 3d, has 5 orbitals, therefore, max of 5 x 2 = 10 electrons .
Full ground state electron configuration
Electrons are filled up from the lowest energy level to the highest. Since we have 17 electrons and now that we know max number of electrons each sublevel can hold, we'll start from 1s and work our way up. Stop when you reach 17 electrons:
Notice the number of electrons are written on top right of each sublevel. So, if you add up all of the superscript numbers, you'll get 2 + 2 + 6 + 2 + 5 = 17 electrons.
Abbreviated ground state electron configuration
- Find noble gas in previous period - Cl is in period 3, Ne is the noble gas in period 2. => [Ne]
- The remaining 7 electrons will reside in
#3s^2 3p^5# . - Abbreviated ground state electron configuration:
#"[Ne]"3s^2 3p^5#

