What must an element be in order to replace another element in a compound?
2 Answers
- In an ionic compound, an element must be able to lose or gain electrons with another element in order to obey the octet rule (to have eight electrons in its valence shell).
Note: Ionic compounds form between a metal and a nonmetal.
- In a covalent compound, an element must be willing to share electrons in order to obey the octet rule (to have eight electrons in its valence shell).
Note: Covalent compounds formed between non-metal and nonmetal
For Example: In this double displacement reaction
The cation from reactant one swap with reactant two.
-
The relationship between sodium and chlorine can occur because a sodium atom needs to lose one electron to become stable and a chlorine atom needs to gain one electron to become stable thus producing an ionic compound.
-
The relationship between hydrogen and sulfur can occur because a hydrogen atom needs to lose one electron and a sulfur atom needs to gain two electrons thus you will need two hydrogen atoms in order to obey the octet rule to produce an ionic compound.
Best Of Luck
-AN
The element that replaces another element in a compound must be more reactive.
Explanation:
This question is about single replacement reactions in which a metal replaces another metal in a compound, or a halogen replaces another halogen in a compound.
The metal or halogen that replaces the metal or halogen in a compound must be more reactive than the metal or halogen replaced.
The diagram below is an activity series of metals and halogens. I will provide a couple of example questions to show you how to use the activity series (sometimes called a reactivity series).
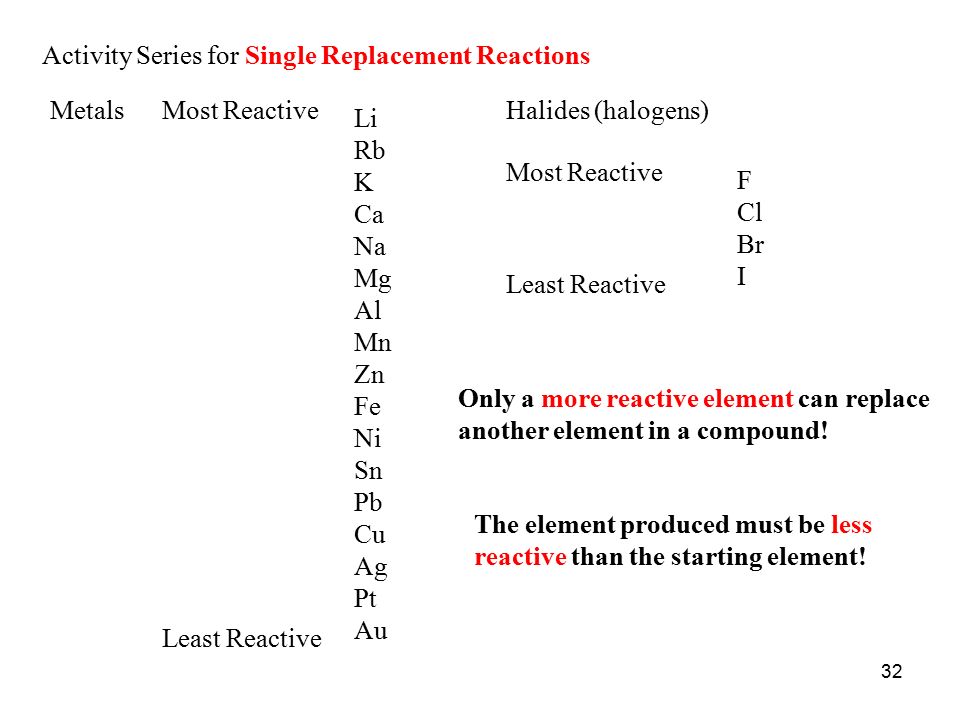 https://slideplayer.com/slide/7603643/
https://slideplayer.com/slide/7603643/
Example 1: Will the following single replacement occur?
Answer:
This reaction will not occur. According to the activity series, aluminum is higher than copper in the series, so it is more reactive and will replace copper in the copper(II) sulfate solution.
Example 2: Will the following single replacement occur?
Answer:
This reaction will not occur because iodine is lower than chlorine on the activity series for halogens. This reaction should be written as:


