Question #497fa
1 Answer
Yes, you got a couple of them wrong. Here's how they should be labeled
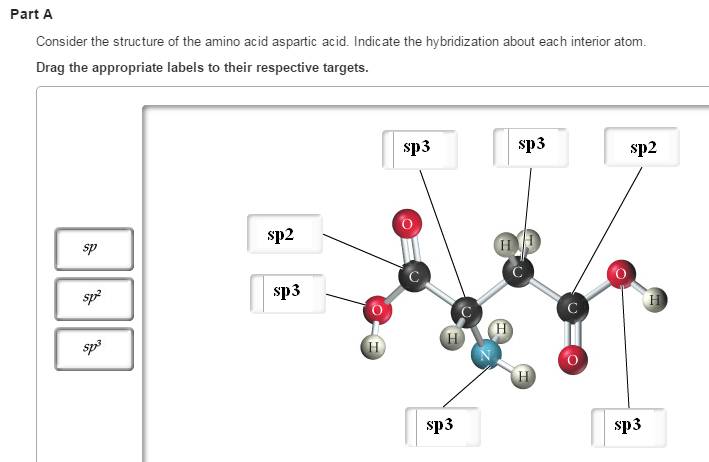
Hybridization is all about regions of electron density that surround an atom - this is referred to as steric number. In carbon's case, these regions of electron density will be bonds, single or double.
In oxygen's case, however, you'll also have lone pairs present, which, as you can see, are not depicted on the structure. The same goes for nitrogen.
For example, an atom that has a steric number equal to 4 will be
Starting from the left side of the molecule
-
Oxygen - it's bonded to two atoms and has 2 lone pairs
#-># SN = 4#-># #sp^3# hybridized; -
Carbon - it's bonded to one ogyxen via a single bond, to another oxygen via a double bond, and to another carbon
#-># SN = 3#-># #sp^2# hybridized; -
Carbon - bonded to two hydrogens, another carbon, and a nitrogen
#-># SN = 4#-># #sp^3# hybridized; -
Nitrogen - bonded to a carbon, two hydrogens, and has 1 lone pair present
#-># SN = 4#-># #sp^3# hybridized; -
Carbon - bonded to two other carbons and two hydrogens
#-># SN = 4#-># #sp^3# hybridized; -
Carbon - identical to the first carbon we encountered
#-># SN = 3#-># #sp^2# hybridized; -
Oxygen - identical to the first oxygen we encountered
#-># SN = 4#-># #sp^3# hybridized;



