Question #8624c
1 Answer
Sulfur dioxide has
To get the hybridization of the central atom, you need to determine how many regions of electron density surround it in the molecule - this is known as the steric number.
Regions of electron density are lone pair of electrons, and bonds, regardless of the type - single, double, and triple bonds all count as 1 region of electron density.
In sulfur dioxide's case, the sulfur atom is bonded to two oxygen atoms and has 1 lone pair of electrons present. This means that its steric number is equal to 3, so it will have 3 hybrid orbitals formed from one s-orbital and two p-orbitals
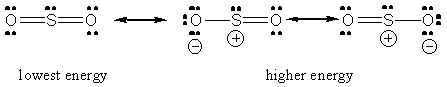
Regardless of which Lewis structure you draw for the sulfur dioxide molecule, the central atom is still bonded to two oxygen atoms and has one lone pair present.
Read more about sulfur dioxide's hybrid structure here:
http://socratic.org/questions/what-is-the-lewis-structure-for-so2

