Question #25404
1 Answer
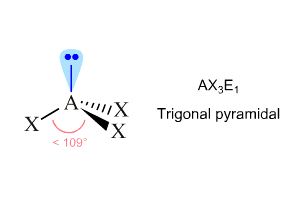
It's molecular geometry is trigonal pyramidal, not trigonal planar.
Explanation:
Check out this answer on the molecular geometry of arsenic trichloride
The idea here is that you have two kinds of geometries, molecular geometry and electron geometry.
Molecular geometry does not take into account any lone pairs that are present on the central atom. In the case of arsenic trichloride, the central arsenic atom has one lone pair of electrons present
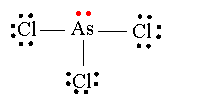
This means that its electron geometry, which does take into account lone pairs, will be tetrahedral, since you essentially have four regions of electron density, three single bonds and one lone pair, that surround the central atom.
The molecular geometry, however, will not be tetrahedral. Moreover, the presence of that lone pair affects the way the three bonds can be arranged in space through repulsion, which means that it will not be trygoinal planar either.
Lone pairs of electrons are said to take up more space than bonding electrons, which means that the three bonds will be pushed away from the lone pair.
The resulting molecular geometry, which indeed corresponds to
Here is a video which discusses VSEPR theory and the shapes of molecules.
Video from: Noel Pauller


