In which of the following compounds does the central atom exhibit #"sp"^2# hybridization?
#"CO"_2#
#"CO"#
#"SO"_2#
#"N"_2"O"#
1 Answer
Sulfur dioxide,
Explanation:
The first thing to focus on is what it means for an atom to be
This type of orbital hybridization implies that the central atom uses three hybrid orbitals, one
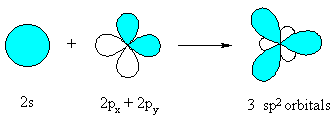
On a more general note, in order for an atom to be
So, take a look at the Lewis structures for your four compounds
- carbon dioxide,
#"CO"_2#
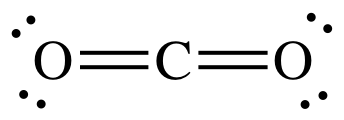
Notice that the central carbon atom forms two double bonds with the two oxygen atoms. Single, double, and triple bonds all count as one region of electron density.
This means that the carbon atom uses two hybrid orbitals, one
- carbon monoxide,
#"CO"#
Notice that the carbon atom is once again surrounded by two regions of electron density - a triple bond and a lone pair of electrons.
This means that it will use two orbitals, one
two
- Sodium dioxide,
#"SO"_2#
The sodium dioxide molecule exhibits resonance, which is another way of saying that it can be represented by using more than one Lewis structure.
However, as far as hybridization of the central atom is concerned, you can use either one.
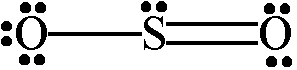
This time, the central sulfur atom is surrounded by three regions of electron density - a single bond, a double bond, and a lone pair of electrons.
This means that the sulfur atom will use three orbitals, one
- Nitrous oxide,
#"N"_2"O"#
The same is true for the nitrous oxide molecule, it exhibits resonance. Once again, any of the resonance structures will work
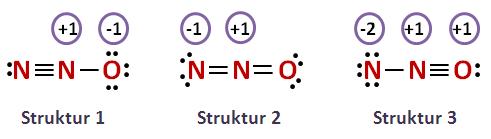
In all three structures, the central nitrogen atom is surrounded by two regions of electron density, which implies that it is

