What is the hybridization of the central phosphorus atom in phosphorus pentafluoride?
1 Answer
Explanation:
In order to determine the hybridization of the central phosphorus atom in phosphorus pentafluoride,
The molecule will have a total of
The phosphorus atom will be the molecule's central atom. It will form single bonds with the five fluorine atoms.
These will account for
The molecule's Lewis structure will look like this
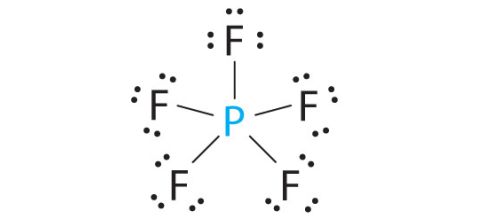
Now you need to focus on the central atom, more specifically on how many regions of electron density surround the central atom - this is known as the steric number
A region of electron density can be a covalent bond - single, double, and triple bonds all count as one region of electron density - or a lone pair of electrons.
Notice that phosphorus is bonded to five fluorine atoms and has no lone pairs of electrons attached, which means that it is surrounded by a total of five regions of electron density, which is equivalent to saying that it has a steric number equal to
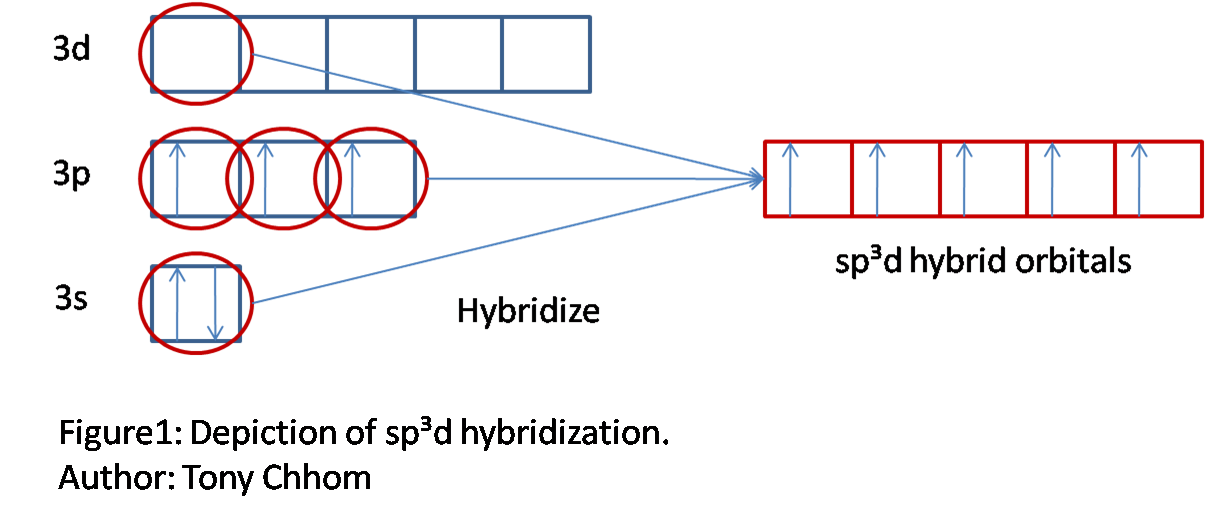
The steric number gives you the number of hybrid orbitals. In this case, a steric number of
- one s-orbital
- three p-orbitals
- one d-orbital
As a result, the central atom will be