Why do tertiary alkyl halides react in an #"S"_N1# mechanism more easily than #"S"_N2#?
1 Answer
Because they are bulky (kinetically stable), and hence block against
They also form the most thermodynamically stable carbocation.
Nucleophilicity is a kinetic phenomenon, so a good nucleophile is fast.
- For
#"S"_N1# reactions of alkyl halides, the#stackrel("central carbon")overbrace("C")-stackrel("leaving group")overbrace"LG"# bond is weak, blocked, and the nucleophile is slow. So the#"LG"# leaves first in an#"S"_N1# fashion, giving a first-order process.
Also, because tertiary carbocations are among the most thermodynamically stable, this departure is favorable (not nonspontaneous).
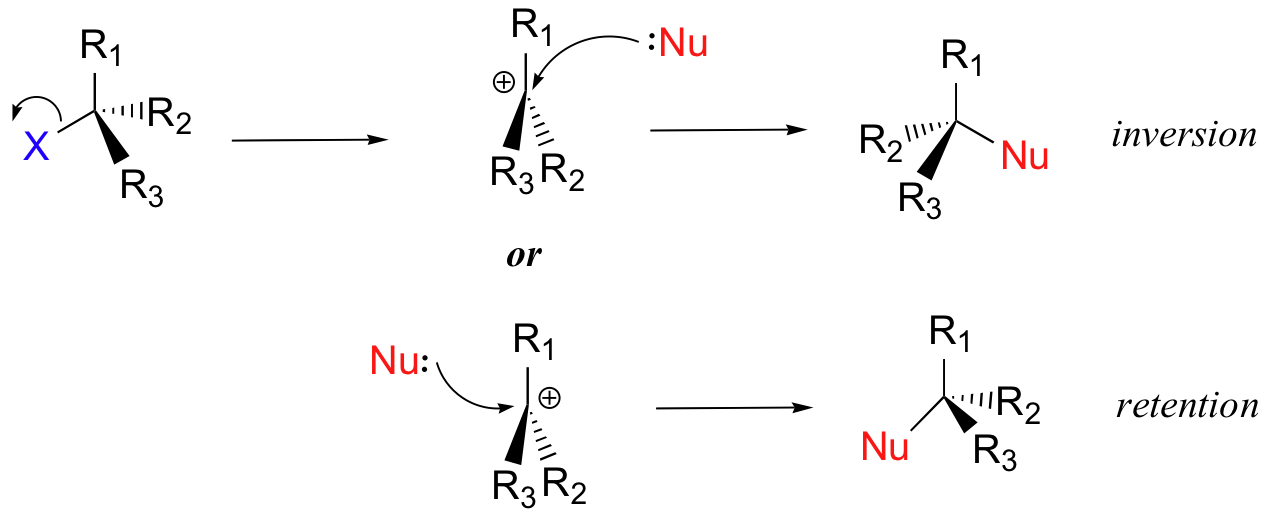
- For
#"S"_N2# reactions of alkyl halides, the#stackrel("central carbon")overbrace("C")-stackrel("leaving group")overbrace"LG"# bond is weak, open, and the nucleophile is fast. So the nucleophile influences the#"LG"# 's leaving.
As less substituted carbocations are less thermodynamically stable, the leaving group cannot easily depart on its own, so the joint process makes it a second-order process.
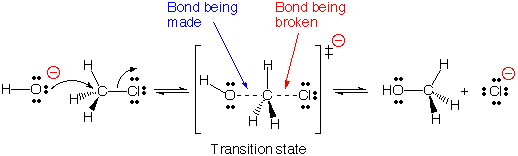
A bulky alkyl halide like a
Basically, the site to be attacked is heavily cluttered, which is hard to get past, and the significant thermodynamic stability of the carbocation promotes its formation during the

