Question #7f20c
1 Answer
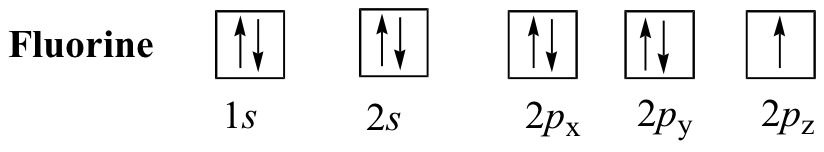
Magnesium. Fluorine.
Explanation:
The thing to remember here is that for main-group elements, the group number also gives you the number of valence electrons.
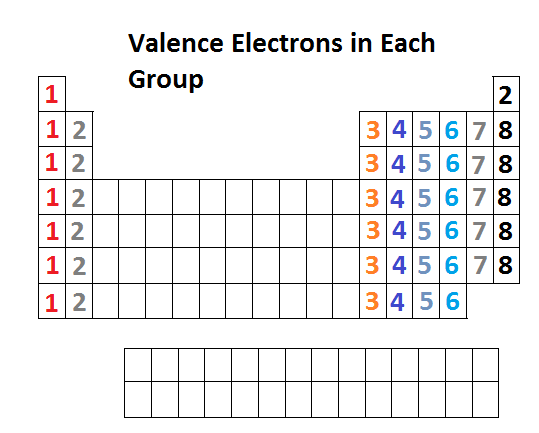
This means that any element located in group 2 will have two valence electrons, and that regardless of the period it's located in.
A quick look in the periodic table will reveal that the element located in period 3, group 2 is magnesium ,
Now, for main-group elements, a period of the periodic table corresponds to the energy level on which an element's valence electrons are located.
The maximum number of electrons that can be added to each energy level,
#color(blue)(|bar(ul(color(white)(a/a)"no. of e"^(-) = 2n^2color(white)(a/a)|)))#
Your unknown element is said to have an electron configuration of
#"2 e"^(-) -># on the first energy level
#"7 e"^(-) -># on the second energy level
The second energy level can hold a maximum of
#"no. of e"^(-) = 2 * 2^2 = "8 e"^(-)#
In your case, the second energy level holds
This is equivalent to saying that the element has
Another quick look in the periodic table will reveal that you're dealing with fluorine,