#"PF"_3# has two things wrong with it. The electron geometry is incorrect, and even the molecular geometry is incorrectly named.
It is trigonal bipyramidal, not "pyramidal" (which does not exist), for the molecular geometry.
It is tetrahedral for the electron geometry.
I assume you are comparing:
- #"CF"_4#
- #"BeBr"_2#
- #"H"_2"O"#
- #"PF"_3#
and that these are neutral molecules, not ions.
GEOMETRY OF #\mathbf("CF"_4)#
Carbon has #color(blue)(4)# valence electrons (its electron configuration is #[He]2s^color(blue)(2) 2p^color(blue)(2)#), so it can share #1# of those #4# with each fluorine, each of which shares #1# of its #color(blue)(7)# (its electron configuration is #[He]2s^color(blue)(2) 2p^color(blue)(5)#).
#"CF"_4# therefore has four electron groups, all of which are bonds, because there are four #"F"# attached.
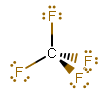
Hence, #"CF"_4# has a tetrahedral electron geometry and molecular geometry.
GEOMETRY OF #\mathbf("BeBr"_2)#
Beryllium has only #color(blue)(2)# valence electrons (#[He] 2s^color(blue)(2)#), so it can only form two electron groups by sharing #1# electron in each. Hydrogen shares its #color(blue)(1)# (#1s^color(blue)(1)#).
Of those two electron groups in #"BeH"_2#, both are bonds, because there are two #"Br"# attached.
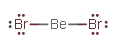
Thus, it has a linear electron geometry and molecular geometry.
GEOMETRY OF #\mathbf("H"_2"O")#
Oxygen has #color(blue)(6)# valence electrons (its electron configuration is #[He]2s^color(blue)(2) 2p^color(blue)(4)#), so it can share #2# of those #6# with hydrogen, leaving #2# lone pairs. Hydrogen shares its #color(blue)(1)# (#1s^color(blue)(1)#).
So, #"H"_2"O"# has four electron groups and two bonds, because it has two #"H"# attached.
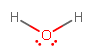
Hence, it has a tetrahedral electron geometry and a bent molecular geometry. This is correct.
GEOMETRY OF #\mathbf("PF"_3)#
Phosphorus has #color(blue)(5)# valence electrons (its electron configuration is #[Ne]3s^color(blue)(2) 3p^color(blue)(3)#), so it can share #3# of those #5# with the fluorines, leaving #1# lone pair. Each fluorine shares #1# of its #color(blue)(7)#.
So, #"PF"_3# has four electron groups and three bonds, because it has three #"F"# attached.
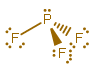
Hence, it has a tetrahedral electron geometry and a trigonal pyramidal molecular geometry.

