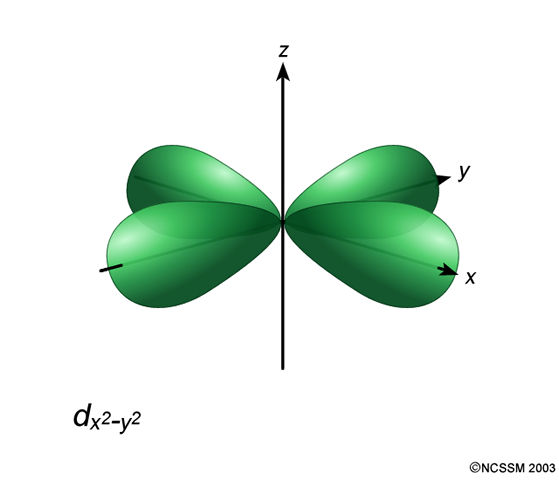
Significance? Well, it corresponds to one of the magnetic quantum numbers in the set #m_l = {-2, -1, 0, +1, +2}#, and has #m_l = -2# by convention.
The #3d_(x^2 - y^2)# orbital is the one lying along the axes:
COMMON BONDING CASES
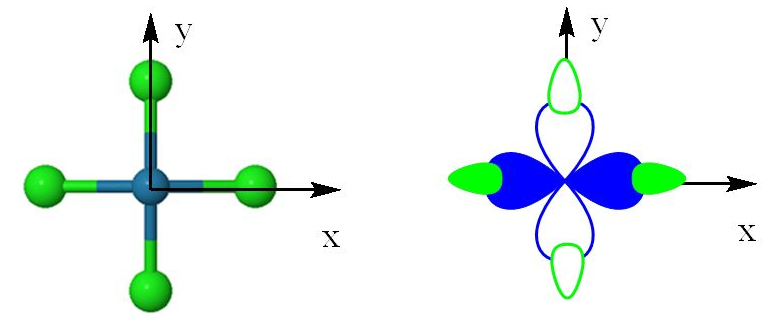
Because of that, it is often used to #sigma# (sigma) bond with surrounding ligands, particularly in a transition metal complex. You'll see a strong contribution from this orbital most often in...
square planar complexes:
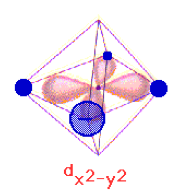
and octahedral complexes:
ENERGIES
In an atom, it is degenerate with the other #"four"# #3d# orbitals, the #d_(z^2)#, #d_(xy)#, #d_(xz)#, and #d_(yz)#.
In a complex, the #d# orbitals split in accordance with their symmetry (horizontal correspondence) and energy (vertical separations) with respect to the other #d# orbitals:
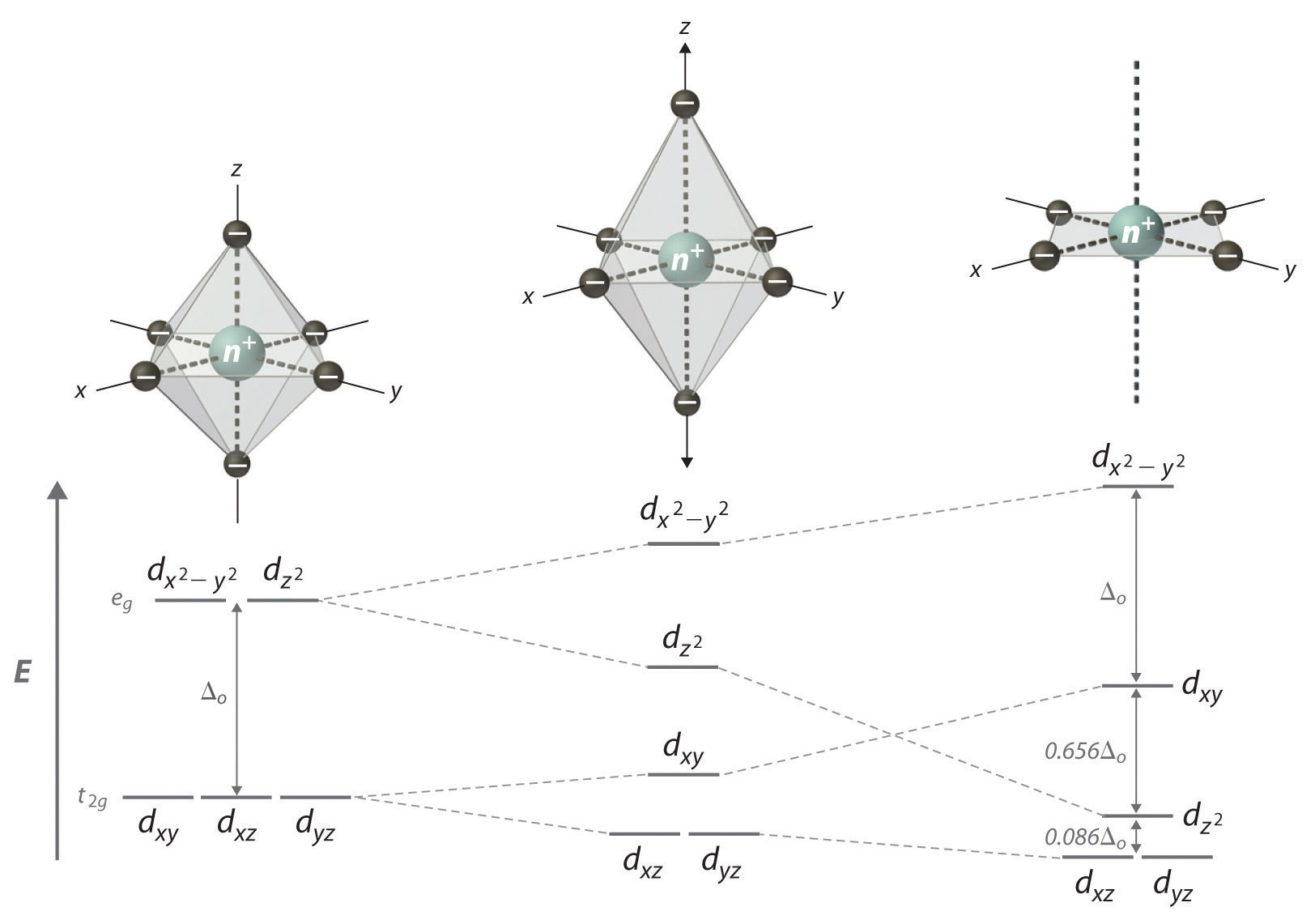
(For the octahedral complex, the symmetries from top to bottom were #E_g# and #T_(2g)#, and for the square planar complex, the symmetries from top to bottom are #B_(1g)#, #B_(2g)#, #A_(1g)#, and #E_g#.)





