How many valence electrons does #"Cr"# have?
1 Answer
The valence electrons of chromium include its
Its electron configuration as an atom is
There most certainly cannot be
For chromium, it is enough of a stabilization to maximize its total spin state by having all unpaired electrons in a
#ul(uarr color(white)(darr))#
#-"7.46 eV"#
#ul(uarr color(white)(darr)) " " ul(uarr color(white)(darr)) " " ul(uarr color(white)(darr)) " " ul(uarr color(white)(darr)) " " ul(uarr color(white)(darr))#
#" "" "" "" "" "-"10.75 eV"#
If other websites tell you that the electron configuration is otherwise, they're not correct, because all textbooks I've ever read in 4 years of college tell me
There is clear evidence that chromium's orbitals need to be able to hold
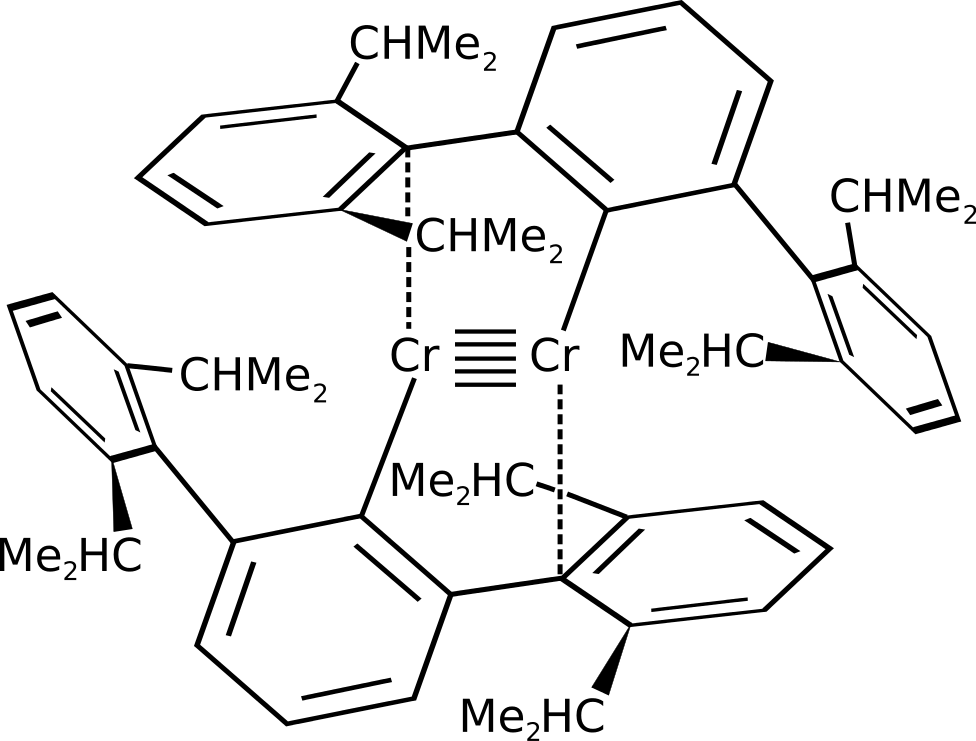
(You can count six bonds and one interaction per chromium atom.)
So it needs to use
If chromium can bond like this, then it must always have the capacity to bond like this, so it wouldn't make sense if it could use all
Therefore, its valence electrons must include those in the

