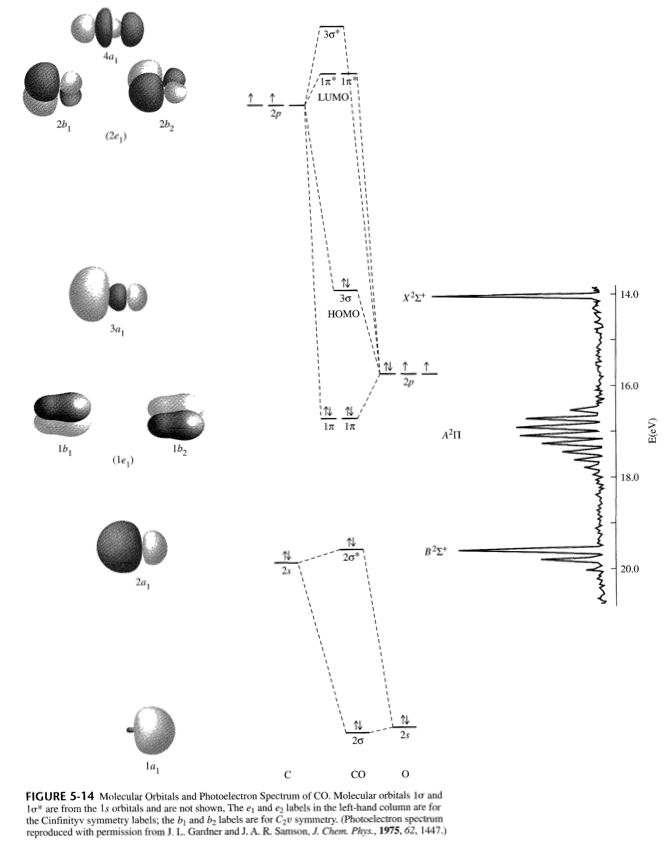
How do I draw the MO diagrams for #"O"_2^-# and #"CO"^(+)# and find out which one is paramagnetic?
1 Answer
Well, the MO diagrams for
- For
#ul("O"_2^(-))# , just take the MO diagram of#"O"_2# and add one electron into the#pi_(2px)^"*"# antibonding molecular orbital. - For
#ul("CO"^(+))# , just take out one of the electrons from the#3sigma# HOMO from the MO diagram of#"CO"# .
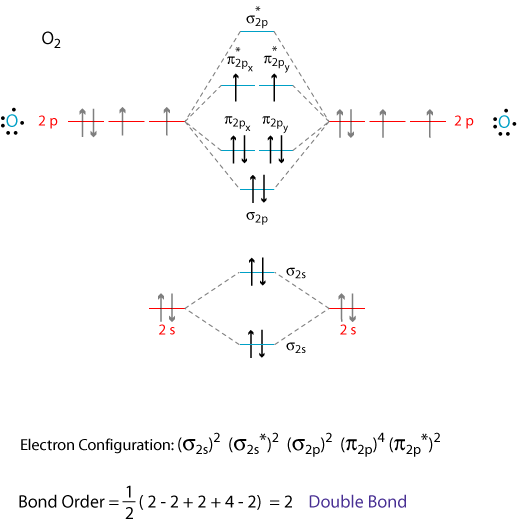
Both substances are paramagnetic, as they have at least one unpaired electron in a given orbital.
The unpaired electron orients itself in the direction of an applied magnetic field, and gives rise to a total magnetic moment
- the total electron spin
#S = |sum_i m_(s,i)|# - the total orbital angular momentum
#L = |sum_i m_(l,i)|#
for the
The total magnetic moment is a value that can be obtained experimentally from magnetic susceptibility measurements.