Is the nonbonding orbital of #"NH"_3# a #p# orbital?
1 Answer
MOSTLY true. The lone pair is in a molecular orbital that is similar to a
Consider this orbital diagram of
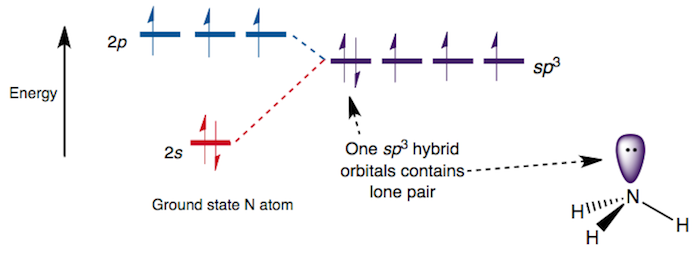
In a sense, I suppose the lone pair is in a
- In a hybridized treatment, one would place the nitrogen lone pair in an
#sp^3# orbital, as seen in the above diagram. In this case, all four orbitals nitrogen uses for bonding are identical, with#75%# #p# character and#25%# #s# character. - In an unhybridized treatment, one would place the nitrogen lone pair in a molecular orbital with significant
#p# characteristics, and the molecular orbital would primarily belong to the nitrogen.
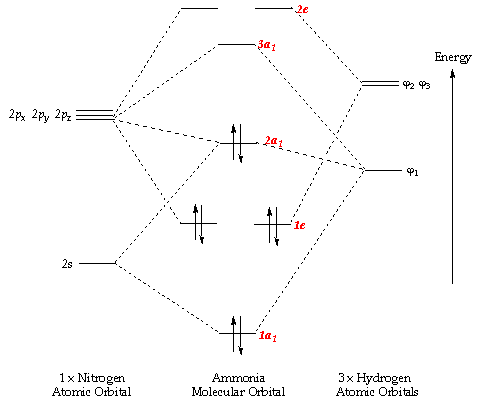
And in the above molecular orbital diagram, I would be referring to the molecular orbital labeled
#2a_1# , which you can see involves interactions between more than one orbital.Therefore, it is not a pure, nonbonding atomic orbital.
Either approach leads to the conclusion that the orbital contains significant

