Are alkyl halides sterically hindered?
1 Answer
Sometimes.
Explanation:
This depends on the specific alkyl halide. For example, take tert-butylbromide and bromopentane.
tert-butylbromide:
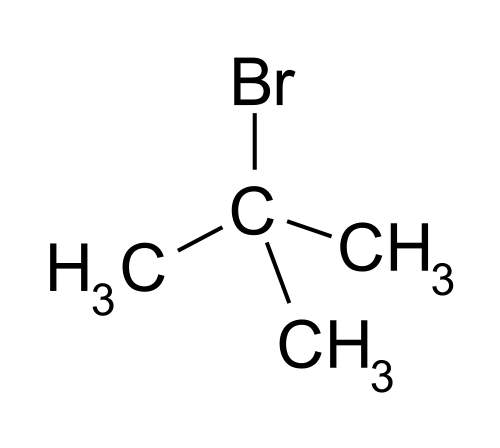
We see that this alkyl halide is tertiary
#(3^o)# , making it very sterically hindered. This makes a backside attack, as seen in the#S_N2# mechanism, virtually impossible. Inversely, the#S_N1# mechanism, for example, would favor this alkyl halide well under the appropriate reaction conditions.
Bromopentane:
This is a primary
#(1^o)# alkyl halide, which has minimal sterical hinderance. This makes a backside attack very possible, and an#S_N2# mechanism would be favored.
Backside attack:
For an

Note this stereochemistry could be flipped. I simply chose one possible orientation for the example.



Note the inversion of stereochemistry, a product of the backside attack. Of course,
#Na^+# is present in solution. It is a counter ion and has not been shown.
For tert-butylbromide, the backside attack isn't plausible, as there are too many other substrates bonded to the same carbon as the halide. It is literally too crowded for this mechanism to take place.
Note that I only considered
Note:
It is possible to have a primary alkyl halide which is too sterically hindered for an
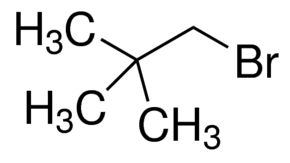
The sterical hinderance of the adjacent carbon is enough to render an

