Are all alkenes and alkynes unsaturated hydrocarbons?
1 Answer
Yes, alkenes and alkynes are both classified as unsaturated hydrocarbons.
Saturation refers to the number of hydrogens attached to each carbon in a molecule. In general, for
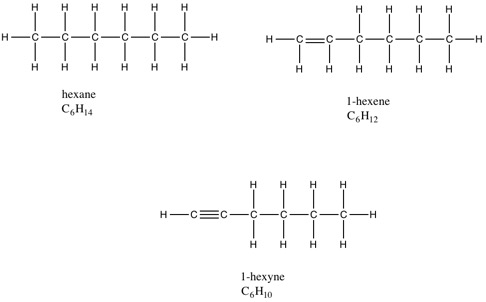
Looking at the structures, we see that only hexane has the full 14 hydrogens. 1-hexene is missing two hydrogens and 1-hexyne is missing four hydrogens. Therefore, both hexene and hexyne are unsaturated hydrocarbons; we say that 1-hexene has one degree of unsaturation and 1-hexyne has two degrees of unsaturation.
In general, the following equation can be used to determine *degrees of unsaturation * (DoU) for a given molecule. As a reference point, anything with more than zero degrees of unsaturation is technically unsaturated.
C - number of carbon atoms
N - number of nitrogen atoms
X - number of halide atoms
H - number of hydrogen atoms
(Oxygen and sulfur do not generally effect the DoU formula)

