Introduction to Reactions and Mechanisms
Key Questions
-
Answer:
Following are the uses of alkanes and alkenes:-
Explanation:
-
Alkanes are saturated hydrocarbons which are formed by single bonding between the carbon atoms. They are mostly used for heating, cooking, and electricity generation. The alkanes which have a higher number of carbon atoms are used for surfacing roads.
-
Alkenes or unsaturated hydrocarbons are formed by double or triple bonding between carbon atoms. They are used for manufacturing of plastic or plastic products.
Now citing the individual uses of the alkanes and the alkenes by naming their different components:-
-
Methane, a form of saturated hydrocarbon, is used for the generation of CNG or Compressed Natural Gas.
-
A mixture of propane and butane is used in LPG cylinders.
-
The manufacturing of polythene uses ethene.
-
-
Let's consider a comparison between the two transition states (alkene vs. alkyne) of a typical electrophilic addition reaction. When you do these, one way to catalyze them is with an acid, so let's look at the first few steps of the acid-catalyzed hydration of an alkene vs. an alkyne:
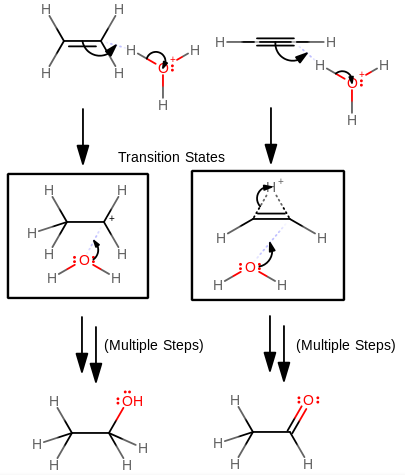
(form of the transition state from Organic Chemistry, Paula Yurkanis Bruice)You can see that for the transition state of the alkyne, the hydrogen is not entirely bonded; it is "complexing" with the double bond, forming a
#\mathbfpi# complex; "idle", until something breaks the interaction (the nucleophilic attack of the water) to get the molecule out of its unstable state.The complex is shaped like a cyclopropane analog, which is highly strained. Also, the high electron density in the double bond makes for some immensely disruptive repulsions that destabilize the transition state.
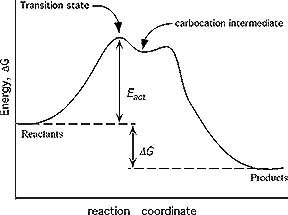
This combination of a highly-strained ring structure and high electron density in the intermediate (transition state) makes alkynes less reactive than alkenes in electrophilic addition reactions. Pictorially, the energy of the transition state is higher on the reaction coordinate diagram.