Can someone explain how do I set up a molecular orbital diagram?
1 Answer
I will stick to diatomic molecules for simplicity. Some general steps are:
- Choose the set of atomic valence orbitals that each atom comes in with. Assume core orbitals don't interact. Determine your coordinate axes.
- Have an approximate idea of the relative orbital energies if working with a heteronuclear diatomic molecule.
- By conservation of orbitals, each of the two corresponding atomic orbital interacts to produce one bonding and one antibonding molecular orbital in the middle.
- By conservation of electrons, the valence electrons that are contributed by each atom equal the total number of valence electrons distributed in the MO diagram.
- Fill the diagram with electrons as usual in accordance with the Aufbau principle, Hund's rule, etc.
Some general rules or tips are:
- Antibonding orbitals usually are approximately as higher in energy as bonding orbitals are lower in energy than the original atomic orbital energies.
- Nonbonding orbitals have either a similar energy to the original atomic orbital energy, or have both bonding and antibonding contributions from the interacting atomic orbitals.
- Large atomic orbital energy offsets correspond to relative molecular orbital energy offsets. This helps with orbital energy ordering.
Let's construct an MO diagram for
CHOOSE YOUR ATOMIC ORBITALS & AXES
- Carbon has
#2s# and#2p# atomic valence orbitals. - Oxygen has
#2s# and#2p# atomic valence orbitals.
ATOMIC ORBITAL ENERGY DIAGRAM
From the original atomic orbital energies, we then construct the two atomic orbital energy diagrams:
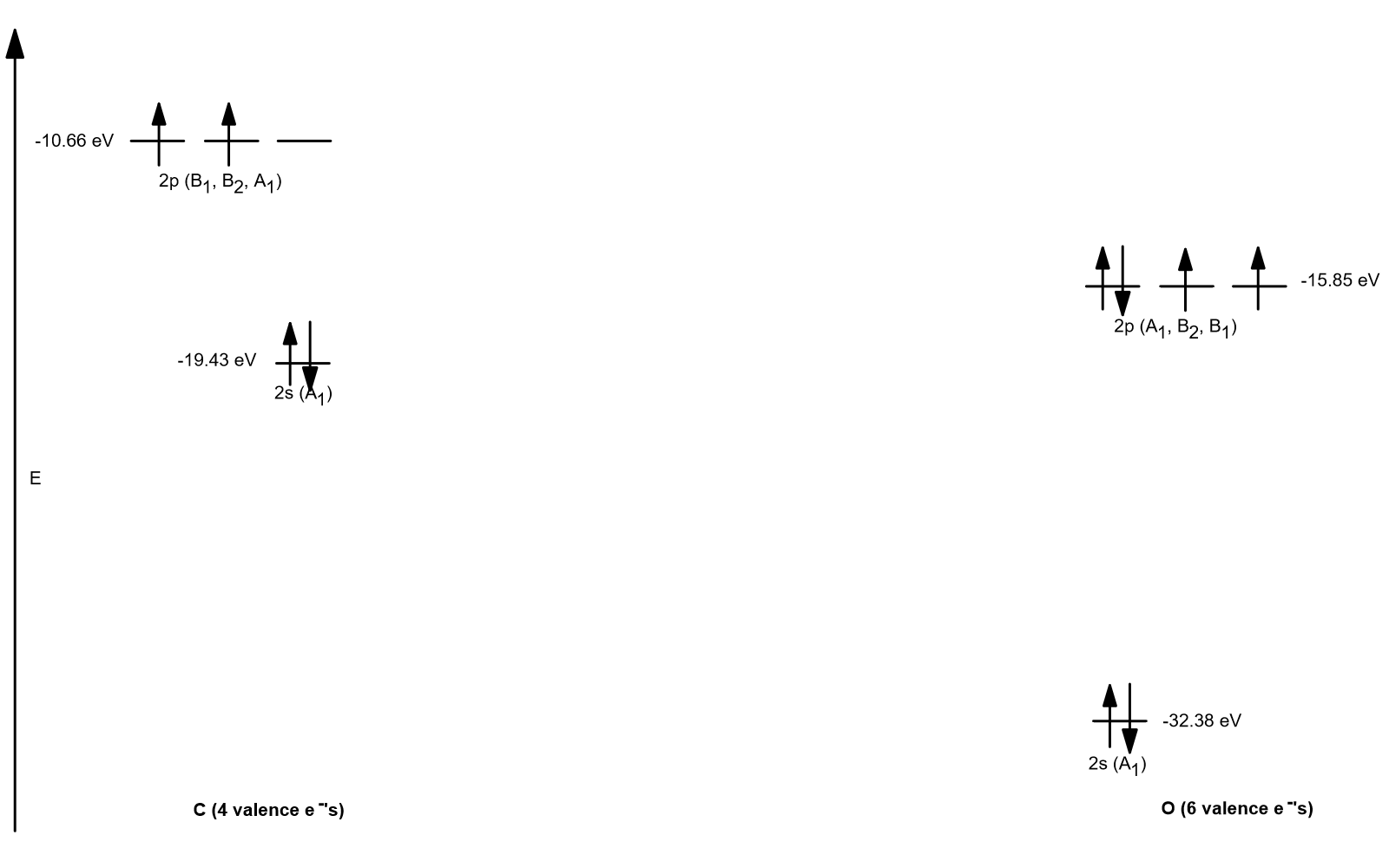
(On an exam, you may only be expected to work with homonuclear diatomic molecules, in which case you probably don't have to worry about relative energies.)
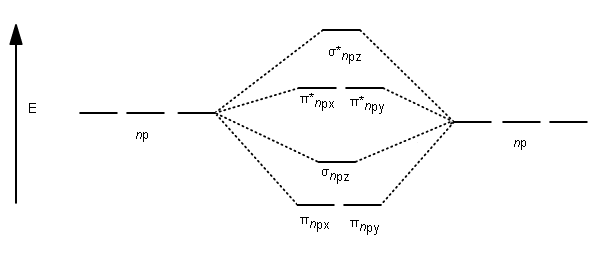
GENERATION OF MOLECULAR ORBITALS
Then, each pair of atomic orbitals generates molecular orbitals as follows. Here is an
For lithium through nitrogen (including nitrogen), orbital mixing occurs, so that the
Therefore, carbon has its energy ordering switched compared to oxygen, and so, the
OVERALL MO DIAGRAM
Now put it together to get:
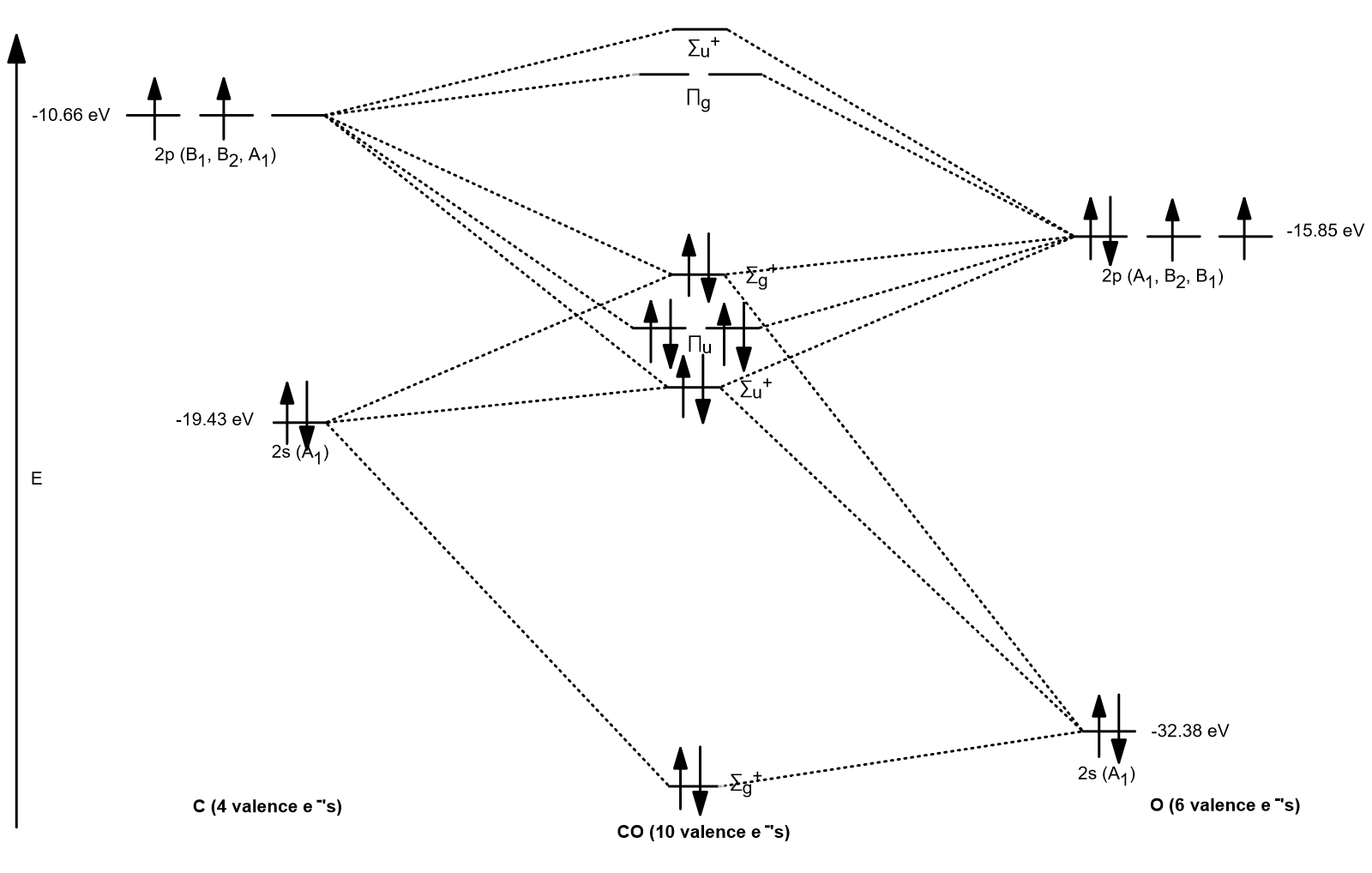
Note that on an exam, you probably aren't expected or required to know exactly how the interactions go. It's enough to know the relative energies of the molecular orbitals compared to the atomic orbitals, and to know how many valence electrons go in.

