Does the cis isomer or the trans isomer of 1,2-dimethylcyclohexane have one methyl group in an equatorial position and the other in an axial position?
1 Answer
That would be the cis isomer, or cis-1,2-dimethylcyclohexane.
If you look at the wedge-dash notation for both cis and trans isomers for 1,2-dimethylcyclohexane, you'll notice a very importance difference between the two
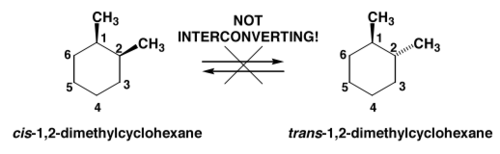
The cis isomer has both methyl groups on wedges, which means that its chair conformers will have both groups in UP position - UP means that the group is placed on a wedge.
The trans isomer has one methyl group on a wedge, or UP, and the other one on a dash, or DOWN. So, even without drawing the chairs for these two isomers you can determine which one will have one methyl on an axial bond and the other on an equatorial bond.
The rule is as follows: for same orientation groups, i.e. both UP or both DOWN, chair placement will be done on alternating axial and equatorial bonds. If you take the numbered carbon atoms in the wedge-dash notations above, groups will be axial, for carbon 1, equatorial, axial, equatorial, axial, and finally equatorial, for carbon 6.
For groups that have alternating orientation, i.e. one UP and one DOWN, chair placement will be made either on axial, or on equatorial, but not alternating between the two.
So, here are two chair conformers for the cis isomer
and for the trans isomer
Notice the placement of the groups. The cis isomer has one methyl group on axial and one on equatorial, both in UP orientation, while the trans isomer has both methyl groups on axial, one in UP and one in DOWN.
Flipping the chairs will not change the orientation of the groups - UP will remain UP and DOWN will remain DOWN, but it will change the bonds on which they're placed - axial will become equatorial and vice versa.
As you can probably see, flipping the chairs will produce a cis conformation that has one methyl on equatorial and the other on axial, and a trans conformation that has both methyl groups on equatorial.
As a conclusion, cis-1,2-dimethylcyclohexane has one methyl group on axial and the other on equatorial.

