What does "trans" isomer mean in cyclohexane ring?
2 Answers
This, for example, can apply to ortho (1,2 connections) compounds. If carbons 1 and 2 have non-hydrogen substituents, trans implies that one substituent is in the back and one is in the front. If both are in front or both are in back, it is cis. Note that it also extrapolates to meta (1,3) and para (1,4) connections.
The trans isomer has the two groups on opposite sides of the cyclohexane ring.
If we imagine the ring to be planar, then one group is above the plane of the ring, and one is below the plane of the ring.
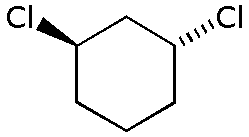
Consider trans-1,3-dichlorocyclohexane.
 web.pdx.edu
web.pdx.edu
If we draw the six carbon atoms in the plane of the paper, one Cl atom is "up" (the wedged bond) and the other is "down" (the dashed bond).
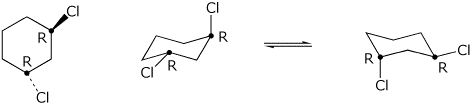
Of course, cyclohexane is not planar but exists mostly in a chair form.
 web.pdx.edu
web.pdx.edu
Even then, we see that the Cl atom on the right is above the plane of the ring and the other is below.


