How can I draw for cis -1-ethyl-3-methylcyclohexane the two chair conformers and indicate which conformer is more stable?
1 Answer
Jul 10, 2015
The 1,3-diequatorial structure is the more stable conformation.
Explanation:
First draw the two chair forms, then add the ethyl and methyl groups to carbons 1 and 3.
The groups must both be on the same side of the ring (both "up" or both "down").
You should get something like this:
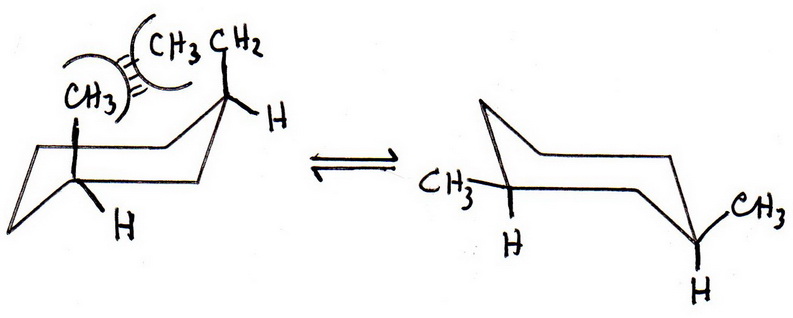
Notice that in the first structure there is a severe 1,3-diaxial interaction between the bulky methyl and ethyl groups.
The electron clouds of the groups are so close that they repel each other (van der Waals repulsion) and introduce strain into the molecule.
In the second structure, both groups are in the equatorial conformation, where they no longer repel each other.
The second (diequatorial) structure is more stable than the first.

