How can I draw for cis -1,2-diethylcyclohexane the two chair conformers and indicate which conformer is more stable?
1 Answer
Start by drawing the wedge-dash notation for cis-1,2-diethylcyclohexane, which looks like this
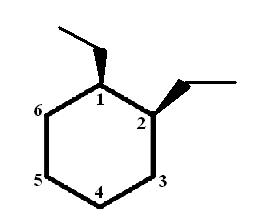
Notice that the two ethyl groups are placed on wedges on carbon (1) and on carbon (2); this is because the molecule is cis, which essentially means that both these groups must be either on wedges, or on dashes. In this case, both are placed on wedges.
In order to draw the first chair conformation you must be familiar with the terms UP and DOWN. For a given group, to be UP means to be on a wedge (coming out of the plane of the page), while being DOWN means to be on a dash (coming out of the plane of the page).
As you can see, both group are in UP position. Now, same orientation groups are placed on alternate axial and equatorial bonds. This means that the first chair conformation will have the ethyl group attached to carbon (1) in UP orientation on an axial bond, and the second ethyl group in UP orientation on an equatorial bond.
Now for the second chair conformation, which is achieved by doing a chair flip. Start with an empty flipped chair template
Now for the groups. When performing a chair flip, it is of the utmost importance to remember that orientation does not change. UP will remain UP and DOWN will remain DOWN. What will change however is the bond positioning - axial will become equatorial and equatorial will become axial.
This means that the ethyl group attached to carbon (1) will go from being UP axial to being UP equatorial. At the same time, the second ethyl group will go from being UP equatorial to being UP axial.
Now for chair stability. Ideally, a chair conformation that has its largest groups in equatorial position will be the most stable. However, both these chairs have one ethyl group in axial position and the other in equatorial position.
This means that both these chair conformations will be equally stable from an energetic standpoint.

