Given #(n-1)d^3 ns^2# and #(n-1)d^5 ns^2#, of the 2 electron configuration which one would exhibit higher oxidation state explain?
1 Answer
Well, oxidation states are hypothetical charges assuming full transfer of a certain number of valence electrons. If the element is capable of losing more valence electrons (when the conditions are right), they can lose that many.
The
That allows all those electrons to be valence if need be. To show how close in energy they are, here are the first-row transition metal energies (Appendix B.9):
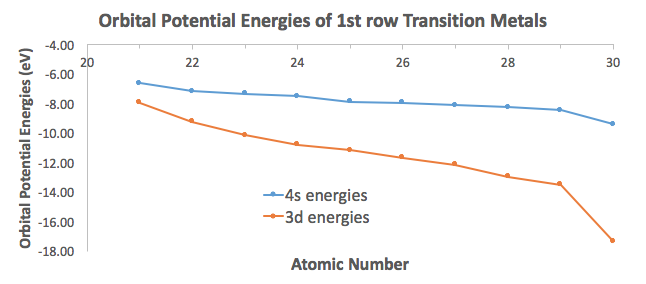
For perspective, the first ionization energy of
Here are representative actual elements with these configurations...
#(n-1)d^color(blue)(3) ns^color(blue)(2)# :#" V"# ,#"Nb"# ,#"Ta"# (#Z = 23, 41, 73# , respectively)
#(n-1)d^color(blue)(5) ns^color(blue)(2)# :#" Cr"# ,#"Tc"# ,#"Re"# (#Z = 25, 43, 75# , respectively)
- The first set do indeed form a maximum oxidation state of
#color(blue)(+5)# , using their#(n-1)d# and#ns# electrons. - The second set do indeed form a maximum oxidation state of
#color(blue)(+7)# , using their#(n-1)d# and#ns# electrons.
And all of their possible oxidation states can be seen here.

