How are epoxides formed?
1 Answer
Jun 25, 2016
A common way to do it is using a peroxyacid on an alkene.
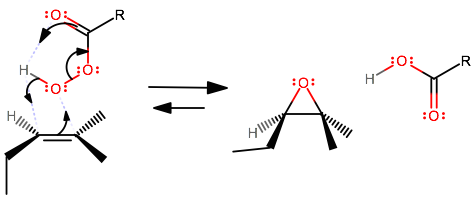
This is a concerted epoxidation mechanism---it happens in one step. These things occur:
- The alkene donates its
#pi# electrons into the bottom oxygen's antibonding orbitals, and receives the bonding electrons from the left#"H"-"O"# bond. This resembles the#\mathbf("Br"_2)# addition onto an alkene. - The carbonyl oxygen intramolecularly donates its
#pi# electrons to grab the peroxy proton within the same peroxyacid. - The bottom oxygen's
#sigma# bond breaks and a#pi# bond forms between the carbonyl carbon and the carboxyl oxygen. This was facilitated through the proton acquisition by the carbonyl oxygen.
Since this is a syn addition of a

