How are pi bonds formed?
1 Answer
Sep 11, 2016
pi bonds are formed by sideways overlap atomic orbitals of bonding atoms.
Explanation:
The formation of pi bonds is illustrated well in the image below,
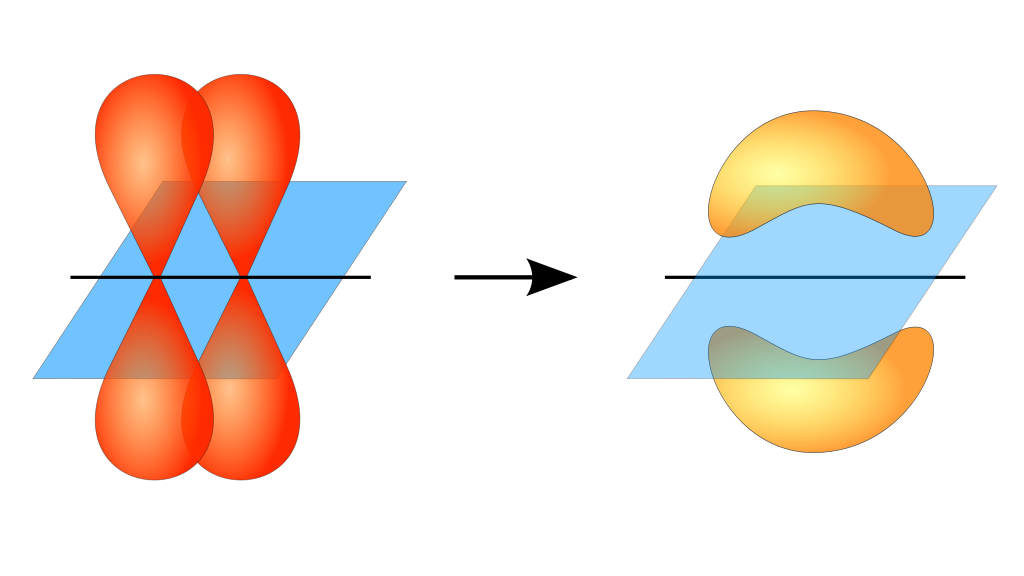
The two p orbitals of the two bonding atoms overlap sideways as shown. The pi electron cloud is perpendicular to the plane of the molecule.
However, sideways overlap is less effective than head to head overlap as in a sigma bond and pi bonds are weaker than sigma bonds.

