Why do single covalent bonds form?
1 Answer
May 20, 2018
Because God wanted it that way...
Explanation:
The modern covalent bond is conceived to be a region of high electron density between two positively-charged atomic nuclei. The equilibrium distance that maximizes attraction between the negatively-charged electron cloud, and the positively-charged nuclei is the equilibrium covalent bond-length...
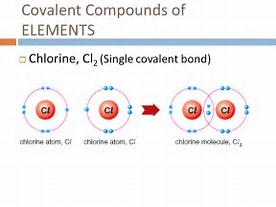
Formation of the bond RESULTS in the release of energy, and so bond-formation is thermodynamically downhill....

