How does a covalent bond affect the stability of atoms?
1 Answer
Dec 18, 2015
It increases their stability; otherwise the bond is not favorable.
You should have seen a diagram similar to this one before:
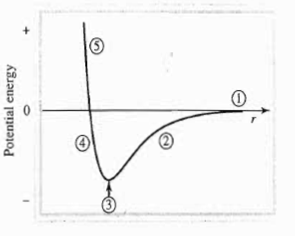
This depicts a general bond-forming process.
- The bond-dissociation limit; this is where the two nuclei are so far apart that either making a bond is not going to happen, or a bond is going to break.
- The nuclei are moving closer together, but aren't quite close enough yet. An attraction is happening between the electrons of atom A and the nucleus of atom B, and vice versa.
- Now the nuclei are at the optimal internuclear distance (separation between two nuclei) for a bond to form.
- This would be when the nuclei are getting closer than they should be; they start repelling each other due to each of their positive charges (remember? Protons can be found in nuclei).
- This is too close; past this point, the repulsion energy is infinitely high, and basically, the nuclei want to move apart because this state is too unstable.
Thus, 3 is the most stable configuration, i.e. it is the location at which the equilibrium geometry can be established and at which there is the greatest possible amount of stability.

