How can I raw dots-and-crosses diagrams (showing outer electrons only) for ammonia, #NH_3#?
1 Answer
Jun 26, 2015
You draw intersecting circles with the dots and crosses in the intersections.
Explanation:
Nitrogen (

Hydrogen (
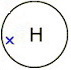
Each atom can get a complete shell if three

Now, each

