How are single covalent bonds made?
1 Answer
The first covalent (single) bond made for any molecule is always done through a head-on overlap of two
(Atomic orbitals are basically regions in the atom where you can find electrons.)
Here's carbon (
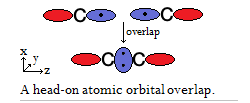
When two orbitals overlap head-on like this, they result in a single bond. A single bond consists of 1 sigma/
Common examples of orbital pairs that overlap to form
Depicted is a
Each covalent single bond consists of two shared valence electrons.
(They are not merely transferred, like they would be in purely ionic bonds.)
In an ideal covalent bond, these electrons are perfectly, evenly shared.

For convenience, we can represent each bond as a line.
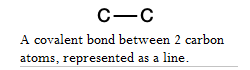
This is particularly useful if you have to draw a very large molecule with many bonds.

