How can I compare the staggered conformer and eclipsed conformers for (ethane) #H_3C- CH_3# in Newman projections?
1 Answer
Jun 14, 2015
In the staggered conformation, the dihedral angle is 60 °. In the eclipsed conformation, the dihedral angle is 0 °.
Explanation:
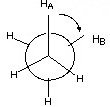
Staggered Ethane
The dihedral angle is the angle between the front and rear bonds.
In the staggered version of ethane, the angle between the
Start by drawing a template for a staggered Newman Projection.
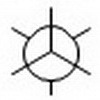
Then add

And you have your staggered Newman projection for ethane.
Eclipsed Ethane
In the eclipsed conformation, the angle behind the front and rear bonds is 0 °( I have off-set the rear bonds slightly so you can see them).
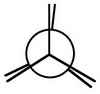
Then add
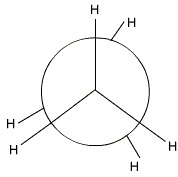
And you have your eclipsed Newman projection for ethane.

