What is the most stable conformer for 3-methylpentane, viewed along the #C_2-C_3# bond using Newman projections?
1 Answer
The most stable conformer for 3-methylpentane viewed along the
So, start with the bond line notation for 3-methylpentane
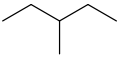
Notice that you have two hydrogens and a methyl group attached to
Start by placing these groups on a Newman projection template. The
Projection 1
All the groups are accounted for: two hydrogen atoms and a methyl group connected to
In order to determine which staggered projection will describe the most stable conformer, you need to draw the other two as well - you do this by rotating the front carbon by
Projection 2 and Projection 3
The most stable conformer has the fewest gauche interactions. The first projection will have two methyl groups in anti positon and a gauche interaction between two methyl groups.
Projection 2 will have two methyl groups in anti postition as well, but this time you have gauche interaction between a methyl and an ethyl group, which is less stable because of the greater repulsion between these two groups.
Projection 3 will be the least stable of the group, since you have gauche interactions between two methyl groups and an ethyl group.
As a conclusion, projection 1 represents the most stable conformer for 3-methylpentane viewed along the

