How can I predict the bond angles for #"TeCl"_4# ?
1 Answer
You can predict the bond angles of tellurium tetrachloride by looking at its molecular geometry.
You can determine its molecular geometry by drawing its Lewis structure. The molecule has a total of 34 valence electrons, 6 from the tellurium atom and 7 from each of the four chlorine atoms.
The tellurium atom will the the central atom of the molecule, the four chlorine atoms being bonded to it through single bonds.
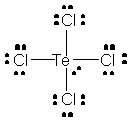
The tellurium atom will also have one lone pair of electrons attached.
The central atom will have a steric number equal to 5, since the tellurium atom is surrounded by five regions of electron density - four single bonds and one lone pair of electrons.
According to VSEPR Theory, the molecule will have a seesaw geometry (
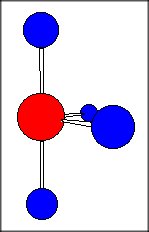
Now for the bond angles. The ideal bond angles for a seesaw molecular geometry are
However, repuslion will cause the lone pair of electrons present on the tellurium to distort these angles a bit by pushing the bonding electrons away from it.
As a result, the bond angles will be less than the ideal values -
SIDE NOTE This is true for a tellurium tetrachloride molecule in the gas phase.

