How can you identify optical isomers?
1 Answer
How do you tell your left hand from right hand; or how do you tell your left shoe from the right shoe?
Explanation:
See this old answer, and also this one with respect to diastereomers.
Some practical tips, given a representation of a chiral centre:
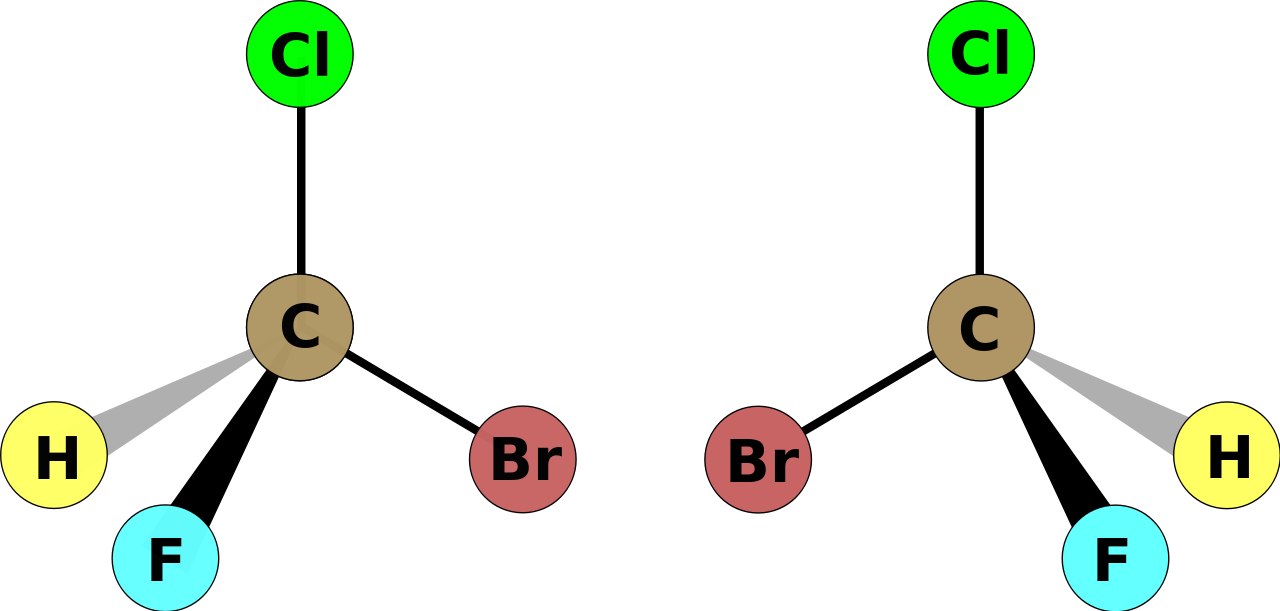
The interchange of ANY 2 substituents at the chiral centre, the carbon nucleus,
Some people find it very easy to vizualize representations of stereoisomers. I am not one of them, and would always require models to inform my reasoning. You should have a play with a set of molecular models in order to establish that what I have said here is kosher. Models are always allowed in organic chemistry tests (and in my experience, they tend to be underutilized). And, as I have mentioned before, you will always find a set of such models on the desks of distinguished professors of organic chemistry. The prof will have a fiddle with the models when an idea strikes him or her.

