How do pi bonds work?
1 Answer
Jun 5, 2016
ORBITAL PERSPECTIVE
Pi (
Either way, this overlap can either be in-phase or out-of-phase.
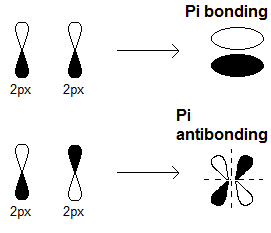
- The in-phase one (same colors overlapping) is lower in energy and is called the bonding
#pi# overlap. It generates a#pi# molecular orbital. - The out-of-phase one (opposite colors overlapping) is higher in energy and is called the antibonding
#pi# overlap. It generates a#pi^"*"# molecular orbital.
A double bond has one
#sigma# and one#pi# bond, while a triple bond has one#sigma# and two#pi# bonds.
MOLECULAR ORBITAL DIAGRAM PERSPECTIVE
The MO diagram depiction is:
where:
#pi_(npx)# is the bonding molecular orbital formed by the in-phase overlap of an#np_x# with an#np_x# atomic orbital.#pi_(npy)# is the bonding molecular orbital formed by the in-phase overlap of an#np_y# with an#np_y# atomic orbital.#pi_(npx)^"*"# is the antibonding molecular orbital formed by the out-of-phase overlap of an#np_x# with an#np_x# atomic orbital.#pi_(npy)^"*"# is the antibonding molecular orbital formed by the out-of-phase overlap of an#np_y# with an#np_y# atomic orbital.
We have three common ways that we can occupy the
- When the
#pi# molecular orbitals are filled but the#pi^"*"# ones are not, we have a#pi# bond. - When both kinds of molecular orbitals are filled, those electrons are nonbonding and are lone pairs.
- When neither kind of molecular orbital is filled, there is no lone pair or bond.
The

