How do you determine if a compound has an aromatic ring?
1 Answer
If you are talking about the experimental identification of a benzene ring, I would use a combination of infrared and NMR spectroscopy.
Explanation:
IR spectroscopy alone may be sufficient to identify an aromatic ring.
Some of the characteristic absorptions are
The overtone and oop absorptions have characteristic appearances depending on the pattern of ring substitution.
Compare the IR spectrum of toluene.
Toluene
(From Quora)
It shows
- Aromatic
"C-H" absorptions atbarν > "3000 cm"^"-1" - 4 weak overtone peaks between
"2000 cm"^"-1" and "1665 cm"^"-1" (monosubstituted phenyl - Strong aromatic absorptions between
"1650 cm"^"-1" and "1450 cm"^"-1" - Strong oop absorptions at
"725 cm"^"-1" and "694 cm"^"-1" (monosubstituted phenyl)
If IR spectroscopy is not conclusive, you can add NMR spectroscopy.
Hydrogens directly attached to an aromatic ring appear at about 7 ppm - 9 ppm, as in the
Toluene
(From rod.beavon.org.uk)
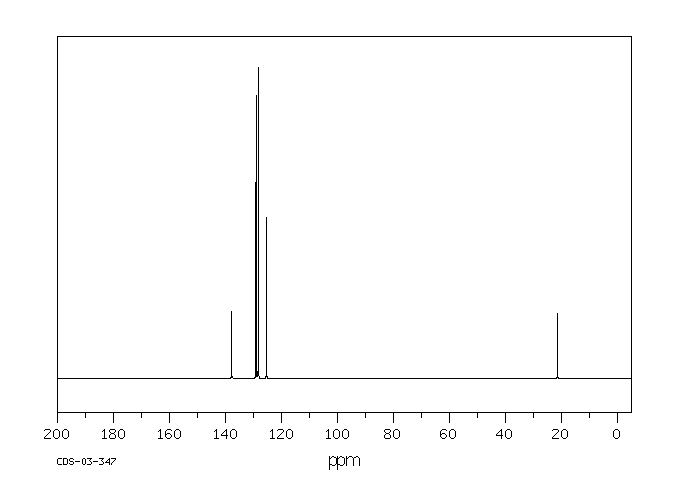
Aromatic carbons usually absorb in the 125 ppm to 150 ppm range, as in the spectrum of toluene below.
 employees.csbsju.edu
employees.csbsju.edu

